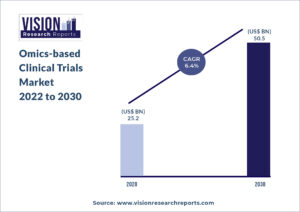
The global omics-based clinical trials market size was valued at USD 25.2 billion in 2020, and is predicted to be worth around USD 50.5 billion by 2030, registering a CAGR of 6.4% during the forecast period 2022 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/39105
Table of Contents
Omics-based Clinical Trials Market Growth Factors
Omics has turned out to be the most advanced approach in molecular research. It includes all the field of biological sciences that ends with the suffix – omics. Various disciplines can be classified as omics such as proteomics, genomics, transcriptomics, and metabolomics. The outbreak of coronavirus accelerated the adoption of new approaches, models, and technology in clinical trials, this has positively impacted market growth.
The demand is growing for a pattern shift toward the stratification of patients or even personalized medicine. There are several complex diseases that urgently need a better understanding of their analysis and more effective therapeutic strategies. Currently, omics-based studies make the majority of precision medicine-based data, e.g. DNA sequencing technique is already being used to identify genetic material that enhances specific cancers.
Omics-based Clinical Trials Market Report Coverage
| Report Scope | Details |
| Market Size | US$ 50.5 billion by 2030 |
| Growth Rate | CAGR of 6.4% From 2022 to 2030 |
| Largest Market | North America |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Phase, Study design, Indication |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | Parexel International Corporation; Pharmaceutical Product Development (PPD); Charles River Laboratory, ICON plc, SGS SA; Eli Lilly and Company; Pfizer Inc.; Covance Inc.; Novo Nordisk; Rebus Bio |
By Phase Analysis
The phase II segment dominated the market for omics-based clinical trials and accounted for the largest revenue share of 37.8% in 2021. The segment is expected to witness a CAGR of 7.1% over the forecast period.
Phase II clinical trials had the highest number of projects in 2020 and this trend is expected to grow further owing to increasing investments in R&D by industry and non-industry sponsors.
By Study Design Analysis
The interventional studies segment dominated the market for omics-based clinical trials and accounted for the largest revenue share of 78.3% in 2021.
The segment is expected to exhibit a CAGR of 7.1% over the forecast period owing to the increasing number of interventional designs of clinical trials. Interventional studies are categorized based on the intervention that is to be studied which includes drug or biologic, surgical procedure, behavioral, and devices.
The expanded access studies segment is anticipated to witness a lucrative CAGR of 8.6% during the forecast period. Interventional study designs are known to provide the most reliable data in epidemiological research in genomics or any other omics-based studies.
By Indication Analysis
The oncology segment dominated the market for omics-based clinical trials and accounted for the largest revenue share of 46.7% in 2021. The segment is expected to witness a CAGR of 7.6% during the forecast period.
The worldwide cancer cases are projected to grow by 50% and worldwide cancer deaths are expected to rise by 60% till 2030 which indicates growing potential for the omics-based clinical trial in this segment.
The research on cardiology is ideally positioned to address the epidemic of noninfectious causes of death, as well as advance the understanding of human health and disease, through the development and implementation of precision medicine.
By Regional Analysis
North America dominated the market for omics-based clinical trials and held the largest revenue share of 43.6% in 2021. This can be attributed to the huge R&D investments, the presence of global players, and their efforts to come up with newer patents.
Asia Pacific is the fastest growing market as many developed nations are investing in Asia-Pacific regions. Besides, the recruitment for clinical trials is increasing in Asia as compared to North America, and Europe. This is due to the large patient pool and low trial cost.
Read also @ Veterinary Telehealth Market to Reach US$ 540.8 Mn by 2030
Major Key Players Covered in The Omics-based Clinical Trials Market Report include
- Parexel International Corporation
- Pharmaceutical Product Development (PPD)
- Charles River Laboratory
- ICON plc
- SGS SA
- Eli Lilly and Company
- Pfizer Inc.
- Covance Inc.
- Novo Nordisk
- Rebus Bio
Omics-based Clinical Trials Market Segmentation
- By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
- By Study Design
- Interventional Studies
- Observational Studies
- Expanded Access Studies
- By Indication
- Oncology
- Cardiology
- Respiratory Diseases
- Skin Diseases
- CNS Diseases
- Immunology
- Genetic Diseases (includes the rare diseases)
- Others (includes ophthalmic, ear diseases, etc)
- Regional
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- Japan
- India
- Australia
- Latin America
- Brazil
- Mexico
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Omics-based Clinical Trials Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Omics-based Clinical Trials Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Omics-Based Clinical Trials Market, By Phase
7.1. Omics-Based Clinical Trials Market, by Phase, 2021-2030
7.1.1. Phase I
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Phase II
7.1.2.1. Market Revenue and Forecast (2017-2030)
7.1.3. Phase III
7.1.3.1. Market Revenue and Forecast (2017-2030)
7.1.4. Phase IV
7.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Omics-Based Clinical Trials Market, By Study Design
8.1. Omics-Based Clinical Trials Market, by Study Design, 2021-2030
8.1.1. Interventional Studies
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Observational Studies
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Expanded Access Studies
8.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Omics-Based Clinical Trials Market, By Indication
9.1. Omics-Based Clinical Trials Market, by Indication, 2021-2030
9.1.1. Oncology
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Cardiology
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Respiratory Diseases
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Skin Diseases
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. CNS Diseases
9.1.5.1. Market Revenue and Forecast (2017-2030)
9.1.6. Immunology
9.1.6.1. Market Revenue and Forecast (2017-2030)
9.1.7. Genetic Diseases (includes the rare diseases)
9.1.7.1. Market Revenue and Forecast (2017-2030)
9.1.8. Others (includes ophthalmic, ear diseases, etc)
9.1.8.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Omics-Based Clinical Trials Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Phase (2017-2030)
10.1.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.1.3. Market Revenue and Forecast, by Indication (2017-2030)
10.1.4. U.S.
10.1.4.1. Market Revenue and Forecast, by Phase (2017-2030)
10.1.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.1.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.1.5. Rest of North America
10.1.5.1. Market Revenue and Forecast, by Phase (2017-2030)
10.1.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.1.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Phase (2017-2030)
10.2.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.2.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2.4. UK
10.2.4.1. Market Revenue and Forecast, by Phase (2017-2030)
10.2.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.2.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2.5. Germany
10.2.5.1. Market Revenue and Forecast, by Phase (2017-2030)
10.2.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.2.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2.6. France
10.2.6.1. Market Revenue and Forecast, by Phase (2017-2030)
10.2.6.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.2.6.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2.7. Rest of Europe
10.2.7.1. Market Revenue and Forecast, by Phase (2017-2030)
10.2.7.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.2.7.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Phase (2017-2030)
10.3.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.3.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3.4. India
10.3.4.1. Market Revenue and Forecast, by Phase (2017-2030)
10.3.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.3.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3.5. China
10.3.5.1. Market Revenue and Forecast, by Phase (2017-2030)
10.3.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.3.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3.6. Japan
10.3.6.1. Market Revenue and Forecast, by Phase (2017-2030)
10.3.6.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.3.6.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3.7. Rest of APAC
10.3.7.1. Market Revenue and Forecast, by Phase (2017-2030)
10.3.7.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.3.7.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Phase (2017-2030)
10.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4.4. GCC
10.4.4.1. Market Revenue and Forecast, by Phase (2017-2030)
10.4.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.4.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4.5. North Africa
10.4.5.1. Market Revenue and Forecast, by Phase (2017-2030)
10.4.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.4.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4.6. South Africa
10.4.6.1. Market Revenue and Forecast, by Phase (2017-2030)
10.4.6.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.4.6.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4.7. Rest of MEA
10.4.7.1. Market Revenue and Forecast, by Phase (2017-2030)
10.4.7.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.4.7.3. Market Revenue and Forecast, by Indication (2017-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Phase (2017-2030)
10.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.5.4. Brazil
10.5.4.1. Market Revenue and Forecast, by Phase (2017-2030)
10.5.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.5.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.5.5. Rest of LATAM
10.5.5.1. Market Revenue and Forecast, by Phase (2017-2030)
10.5.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
10.5.5.3. Market Revenue and Forecast, by Indication (2017-2030)
Chapter 11. Company Profiles
11.1. Parexel International Corporation
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Pharmaceutical Product Development (PPD)
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Charles River Laboratory
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. ICON plc
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. SGS SA
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Eli Lilly and Company
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Pfizer Inc.
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Covance Inc.
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Novo Nordisk
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Rebus Bio
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/39105
Contact Us:
Vision Research Reports
Call: +1 9197 992 333
