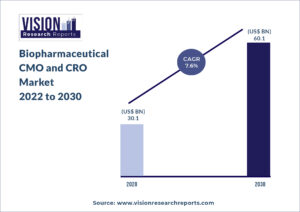
The global biopharmaceutical CMO and CRO market size was valued at USD 30.1 billion in 2020, and is predicted to be worth around USD 60.1 billion by 2030, registering a CAGR of 7.6% during the forecast period 2022 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/39095
Table of Contents
Biopharmaceutical CMO and CRO Market Growth Factors
There was unprecedented growth in 2020 due to the COVID-19 pandemic. The rising investments in the biopharmaceutical industry by the prominent participants to enhance their productivity and efficiency have driven the bio manufacturers to increase their focus on outsourcing activities. Currently, biopharma companies have begun outsourcing the resource and capital-intensive steps and, in a few cases, the entire chain of biomanufacturing, thereby boosting the demand for contract-based services.
The robustness of venture capital investments is one of the important supportive factors that has created opportunities for the growth of CMOs. Venture capital funds are considered more reliable than public equity. An increase in the availability of venture funds for life sciences is expected to bolster the growth of CMOs. However, these CMOs & CROs are expected to face competition from in-house departments of pharmaceutical & biotechnology companies. Several big pharma companies like Novartis announced to maintain and expand their biomanufacturing processes in-house over the coming years.
Biopharmaceutical CMO and CRO Market Report Coverage
| Report Scope | Details |
| Market Size | US$ 60.1 billion by 2030 |
| Growth Rate | CAGR of 7.6% From 2022 to 2030 |
| Largest Market | North America |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Source, Service, Product |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | Boehringer Ingelheim GmbH; Lonza Group AG; Inno Biologics Sdn Bhd; Rentschler Biopharma SE; JRS Pharma; Biomeva GmbH; ProBioGen AG; Fujifilm Diosynth Biotechnologies U.S.A., Inc.; Toyobo Co., Ltd.; Samsung Biologics |
By Source Analysis
The mammalian segment held the highest market share of more than 55% in 2021 owing to the lack of internal expertise in the industry. This is attributed to their capabilities to add human-like post-translational modifications to complex protein therapeutics.
These developments have led to more productive and efficient manufacturing of biologics using mammalian cells. Non-mammalian cell lines, such as microbial cell lines, are recognized as potent factories. Innovative strategies are being implemented to identify and explore the potential of various microbes.
By Service Analysis
The contract research segment is expected to exhibit the fastest CAGR of 7.5% over the forecast period owing to the increasing outsourcing of research activities by biopharmaceutical companies.
These factors have resulted in the largest share of this segment. On the other hand, CROs are striving to capitalize on the potential avenues in the industry.
By Product Analysis
The biologics segment dominated the market with the largest revenue share of over 81% in 2021. This growth is attributed to the high specificity of biologics, complex manufacturing steps, and a higher success rate as compared to other drug molecules.
Several companies are investing in biosimilar development to outperform the safety, efficacy, disposition, or cost of earlier in-class innovator drugs. This has increased the level of competition amongst innovator manufacturers, which in turn, is likely to benefit the CMOs.
By Regional Analysis
North America held the largest revenue share of 33.97% in 2021. This can be attributed to the local presence of several service providers in the region. Also, a significant number of approved products in the U.S. are being manufactured by CMOs.
The key reason for the rise in outsourcing in Asian countries includes cost-associated benefits, such as lower labor costs and operating costs across the region.
Read also @ Pacemakers Market Estimated to Cross US$ 6.01 Bn by 2030
Major Key Players Covered in The Biopharmaceutical CMO and CRO Market Report include
- Boehringer Ingelheim GmbH
- Lonza Group AG
- Inno Biologics Sdn Bhd
- Rentschler Biopharma SE
- JRS Pharma
- Biomeva GmbH
- ProBioGen AG
- Fujifilm Diosynth Biotechnologies U.S.A., Inc.
- Toyobo Co., Ltd.
- Samsung Biologics
Biopharmaceutical CMO and CRO Market Segmentation
- By Source
- Mammalian
- Non-mammalian
- By Service Type
- Contract Manufacturing
- Process Development
- Downstream
- Upstream
- Fill & Finish Operations
- Analytical & QC Studies
- Packaging
- Process Development
- Contract Research
- Oncology
- Inflammation & Immunology
- Cardiology
- Neuroscience
- Others
- Contract Manufacturing
- By Product
- Biologics
- Monoclonal antibodies (MAbs)
- Recombinant Proteins
- Vaccines
- Antisense, RNAi, & Molecular Therapy
- Others
- Biosimilars
- Biologics
- Regional
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- Asia Pacific
- China
- India
- Latin America
- Brazil
- Middle East & Africa
- South Africa
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Biopharmaceutical CMO and CRO Market, By Source
7.1. Biopharmaceutical CMO and CRO Market, by Source, 2021-2030
7.1.1. Mammalian
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Non-mammalian
7.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Biopharmaceutical CMO and CRO Market, By Service Type
8.1. Biopharmaceutical CMO and CRO Market, by Service Type, 2021-2030
8.1.1. Contract Manufacturing
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Contract Research
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Biopharmaceutical CMO and CRO Market, By Product
9.1. Biopharmaceutical CMO and CRO Market, by Product, 2021-2030
9.1.1. Biologics
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Biosimilars
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Biopharmaceutical CMO and CRO Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Source (2017-2030)
10.1.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.1.3. Market Revenue and Forecast, by Product (2017-2030)
10.1.4. U.S.
10.1.4.1. Market Revenue and Forecast, by Source (2017-2030)
10.1.4.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.1.4.3. Market Revenue and Forecast, by Product (2017-2030)
10.1.5. Rest of North America
10.1.5.1. Market Revenue and Forecast, by Source (2017-2030)
10.1.5.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.1.5.3. Market Revenue and Forecast, by Product (2017-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Source (2017-2030)
10.2.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.2.3. Market Revenue and Forecast, by Product (2017-2030)
10.2.4. UK
10.2.4.1. Market Revenue and Forecast, by Source (2017-2030)
10.2.4.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.2.4.3. Market Revenue and Forecast, by Product (2017-2030)
10.2.5. Germany
10.2.5.1. Market Revenue and Forecast, by Source (2017-2030)
10.2.5.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.2.5.3. Market Revenue and Forecast, by Product (2017-2030)
10.2.6. France
10.2.6.1. Market Revenue and Forecast, by Source (2017-2030)
10.2.6.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.2.6.3. Market Revenue and Forecast, by Product (2017-2030)
10.2.7. Rest of Europe
10.2.7.1. Market Revenue and Forecast, by Source (2017-2030)
10.2.7.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.2.7.3. Market Revenue and Forecast, by Product (2017-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Source (2017-2030)
10.3.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.3.3. Market Revenue and Forecast, by Product (2017-2030)
10.3.4. India
10.3.4.1. Market Revenue and Forecast, by Source (2017-2030)
10.3.4.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.3.4.3. Market Revenue and Forecast, by Product (2017-2030)
10.3.5. China
10.3.5.1. Market Revenue and Forecast, by Source (2017-2030)
10.3.5.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.3.5.3. Market Revenue and Forecast, by Product (2017-2030)
10.3.6. Japan
10.3.6.1. Market Revenue and Forecast, by Source (2017-2030)
10.3.6.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.3.6.3. Market Revenue and Forecast, by Product (2017-2030)
10.3.7. Rest of APAC
10.3.7.1. Market Revenue and Forecast, by Source (2017-2030)
10.3.7.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.3.7.3. Market Revenue and Forecast, by Product (2017-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Source (2017-2030)
10.4.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.4.3. Market Revenue and Forecast, by Product (2017-2030)
10.4.4. GCC
10.4.4.1. Market Revenue and Forecast, by Source (2017-2030)
10.4.4.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.4.4.3. Market Revenue and Forecast, by Product (2017-2030)
10.4.5. North Africa
10.4.5.1. Market Revenue and Forecast, by Source (2017-2030)
10.4.5.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.4.5.3. Market Revenue and Forecast, by Product (2017-2030)
10.4.6. South Africa
10.4.6.1. Market Revenue and Forecast, by Source (2017-2030)
10.4.6.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.4.6.3. Market Revenue and Forecast, by Product (2017-2030)
10.4.7. Rest of MEA
10.4.7.1. Market Revenue and Forecast, by Source (2017-2030)
10.4.7.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.4.7.3. Market Revenue and Forecast, by Product (2017-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Source (2017-2030)
10.5.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.5.3. Market Revenue and Forecast, by Product (2017-2030)
10.5.4. Brazil
10.5.4.1. Market Revenue and Forecast, by Source (2017-2030)
10.5.4.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.5.4.3. Market Revenue and Forecast, by Product (2017-2030)
10.5.5. Rest of LATAM
10.5.5.1. Market Revenue and Forecast, by Source (2017-2030)
10.5.5.2. Market Revenue and Forecast, by Service Type (2017-2030)
10.5.5.3. Market Revenue and Forecast, by Product (2017-2030)
Chapter 11. Company Profiles
11.1. Boehringer Ingelheim GmbH
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Lonza Group AG
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Inno Biologics Sdn Bhd
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Rentschler Biopharma SE
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. JRS Pharma
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Biomeva GmbH
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. ProBioGen AG
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Fujifilm Diosynth Biotechnologies U.S.A., Inc.
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Toyobo Co., Ltd.
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Samsung Biologics
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/39095
Contact Us:
Vision Research Reports
Call: +1 9197 992 333
