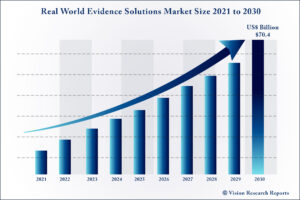
The global Real World Evidence Solutions market size is expected to be worth around US$ 70.4 billion by 2030, according to a new report by Vision Research Reports.
The global Real World Evidence Solutions market size was valued at US$ 38.2 billion in 2020 and is anticipated to grow at a CAGR of 8.6% during forecast period 2021 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/39021
Table of Contents
Real World Evidence Solutions Market Growth Factors
The key factors driving the real world evidence solutions (RWE) market growth include the favorable government regulations and shift from volume to value-based care. Furthermore, the increasing prevalence of chronic disorders is anticipated to positively influence market growth.
Support from regulatory bodies for using real world evidence (RWE) solutions and an increase in R&D spending are anticipated to boost the market growth. Furthermore, the shift from volume to value-based care is expected to fuel market growth.
During the pandemic, companies in the market diverted their focus towards providing solutions for curbing the spread of COVID-19. For instance, IBM collaborated with Salesforce for building digital health passes, which incorporated data, such as vaccine status, temperature checks, and COVID-19 test results for helping organizations to reopen safely.
Real World Evidence Solutions Market Report Coverage
| Report Scope | Details |
| Market Size | US$ 70.4 billion by 2030 |
| Growth Rate | CAGR of 8.6% From 2021 to 2030 |
| Base Year | 2021 |
| Forecast Period | 2021 to 2030 |
| Segments Covered | Component, Application, End user |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | IQVIA; IBM; PPD, Inc.; Parexel International Corp.; PerkinElmer, Inc.; Icon Plc; Oracle; Syneos Health; Cegedim Health Data; Medpace |
By Component Analysis
The services segment accounted for the maximum revenue share of more than 57% in 2020. The market growth is attributed to the high adoption of real-world services by pharmaceutical & biotechnology companies as well as healthcare providers.
The data sets segment consists of clinical settings data, claims data, pharmacy data, and patient-powered data. The segment is anticipated to witness growth owing to arise in the volume of data generated in healthcare facilities and growing attention for additional insights on epidemiology.
By Application Analysis
The drug development and approvals segment accounted for the highest revenue share of more than 29.5% in 2020. Real-world evidence solutions services allow pharmaceutical companies and healthcare providers as well as payers for efficient management of operations and accelerate the process of drug development and its approval.
The reimbursement/coverage and regulatory decision-making segment is expected to grow at the fastest CAGR of more than 8% from 2021 to 2030 owing to the rising focus towards clinical value evidence for making decisions to provide coverage and the rising therapy costs.
By End-user Analysis
The pharmaceutical & medical device companies segment accounted for the maximum revenue share of around 33.9% in 2020. The segment growth is attributed to the rising importance of RWE studies in the drug approval process, assessing drug performance in real-world settings, and preventing drug recalls.
The healthcare payers segment is expected to grow at the fastest growth rate of more than 8.5% over the forecast period owing to the increasing awareness among the payers regarding the importance of medical device/drug safety & their adverse effects and favorable reimbursement scenario, especially in developed countries.
By Regional Analysis
North America accounted for the largest revenue share of 44.4% in 2020. The large share of the region is credited to the presence of key players in the U.S. Rising number of RWE service providers and favorable government regulations in the region are expected to drive the market further.
Asia Pacific is expected to be the fastest-growing regional market during the forecast years owing to the rising government initiatives for the adoption of RWE studies and the presence of many Contract Research Organizations(CROs) and manufacturing companies in countries, such as China and India.
Read also @ 3D Printed Brain Model Market Insight and Trends 2030
Major Key Players Covered in The Real World Evidence Solutions Market Report include
- IQVIA
- IBM
- PPD, Inc.
- Parexel International Corp.
- PerkinElmer, Inc.
- Icon Plc
- Oracle
- Syneos Health
- Cegedim Health Data
- Medpace
Real World Evidence Solutions Market Segmentation
- By Component
- Services
- Data Sets
- Clinical Settings Data
- Claims Data
- Pharmacy Data
- Patient-Powered Data
- By Application
- Drug Development & Approvals
- Medical Device Development & Approvals
- Reimbursement/Coverage & Regulatory Decision Making
- Post Market Safety & Adverse Events Monitoring
- By End-user
- Pharmaceutical & Medical Device Companies
- Healthcare Payers
- Healthcare Providers
- Others
- Regional
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- France
- Italy
- Spain
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- MEA
- South Africa
- Saudi Arabia
- Israel
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Real World Evidence Solutions Market, By Component
7.1. Real World Evidence Solutions Market, by Component, 2021-2030
7.1.1. Services
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Data Sets
7.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Real World Evidence Solutions Market, By Application
8.1. Real World Evidence Solutions Market, by Application, 2021-2030
8.1.1. Drug Development & Approvals
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Medical Device Development & Approvals
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Reimbursement/Coverage & Regulatory Decision Making
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Post Market Safety & Adverse Events Monitoring
8.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Real World Evidence Solutions Market, By End User
9.1. Real World Evidence Solutions Market, by End User, 2021-2030
9.1.1. Pharmaceutical & Medical Device Companies
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Healthcare Payers
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Healthcare Providers
9.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Real World Evidence Solutions Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Component (2017-2030)
10.1.2. Market Revenue and Forecast, by Application (2017-2030)
10.1.3. Market Revenue and Forecast, by End User (2017-2030)
10.1.4. U.S.
10.1.4.1. Market Revenue and Forecast, by Component (2017-2030)
10.1.4.2. Market Revenue and Forecast, by Application (2017-2030)
10.1.4.3. Market Revenue and Forecast, by End User (2017-2030)
10.1.5. Rest of North America
10.1.5.1. Market Revenue and Forecast, by Component (2017-2030)
10.1.5.2. Market Revenue and Forecast, by Application (2017-2030)
10.1.5.3. Market Revenue and Forecast, by End User (2017-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Component (2017-2030)
10.2.2. Market Revenue and Forecast, by Application (2017-2030)
10.2.3. Market Revenue and Forecast, by End User (2017-2030)
10.2.4. UK
10.2.4.1. Market Revenue and Forecast, by Component (2017-2030)
10.2.4.2. Market Revenue and Forecast, by Application (2017-2030)
10.2.4.3. Market Revenue and Forecast, by End User (2017-2030)
10.2.5. Germany
10.2.5.1. Market Revenue and Forecast, by Component (2017-2030)
10.2.5.2. Market Revenue and Forecast, by Application (2017-2030)
10.2.5.3. Market Revenue and Forecast, by End User (2017-2030)
10.2.6. France
10.2.6.1. Market Revenue and Forecast, by Component (2017-2030)
10.2.6.2. Market Revenue and Forecast, by Application (2017-2030)
10.2.6.3. Market Revenue and Forecast, by End User (2017-2030)
10.2.7. Rest of Europe
10.2.7.1. Market Revenue and Forecast, by Component (2017-2030)
10.2.7.2. Market Revenue and Forecast, by Application (2017-2030)
10.2.7.3. Market Revenue and Forecast, by End User (2017-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Component (2017-2030)
10.3.2. Market Revenue and Forecast, by Application (2017-2030)
10.3.3. Market Revenue and Forecast, by End User (2017-2030)
10.3.4. India
10.3.4.1. Market Revenue and Forecast, by Component (2017-2030)
10.3.4.2. Market Revenue and Forecast, by Application (2017-2030)
10.3.4.3. Market Revenue and Forecast, by End User (2017-2030)
10.3.5. China
10.3.5.1. Market Revenue and Forecast, by Component (2017-2030)
10.3.5.2. Market Revenue and Forecast, by Application (2017-2030)
10.3.5.3. Market Revenue and Forecast, by End User (2017-2030)
10.3.6. Japan
10.3.6.1. Market Revenue and Forecast, by Component (2017-2030)
10.3.6.2. Market Revenue and Forecast, by Application (2017-2030)
10.3.6.3. Market Revenue and Forecast, by End User (2017-2030)
10.3.7. Rest of APAC
10.3.7.1. Market Revenue and Forecast, by Component (2017-2030)
10.3.7.2. Market Revenue and Forecast, by Application (2017-2030)
10.3.7.3. Market Revenue and Forecast, by End User (2017-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Component (2017-2030)
10.4.2. Market Revenue and Forecast, by Application (2017-2030)
10.4.3. Market Revenue and Forecast, by End User (2017-2030)
10.4.4. GCC
10.4.4.1. Market Revenue and Forecast, by Component (2017-2030)
10.4.4.2. Market Revenue and Forecast, by Application (2017-2030)
10.4.4.3. Market Revenue and Forecast, by End User (2017-2030)
10.4.5. North Africa
10.4.5.1. Market Revenue and Forecast, by Component (2017-2030)
10.4.5.2. Market Revenue and Forecast, by Application (2017-2030)
10.4.5.3. Market Revenue and Forecast, by End User (2017-2030)
10.4.6. South Africa
10.4.6.1. Market Revenue and Forecast, by Component (2017-2030)
10.4.6.2. Market Revenue and Forecast, by Application (2017-2030)
10.4.6.3. Market Revenue and Forecast, by End User (2017-2030)
10.4.7. Rest of MEA
10.4.7.1. Market Revenue and Forecast, by Component (2017-2030)
10.4.7.2. Market Revenue and Forecast, by Application (2017-2030)
10.4.7.3. Market Revenue and Forecast, by End User (2017-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Component (2017-2030)
10.5.2. Market Revenue and Forecast, by Application (2017-2030)
10.5.3. Market Revenue and Forecast, by End User (2017-2030)
10.5.4. Brazil
10.5.4.1. Market Revenue and Forecast, by Component (2017-2030)
10.5.4.2. Market Revenue and Forecast, by Application (2017-2030)
10.5.4.3. Market Revenue and Forecast, by End User (2017-2030)
10.5.5. Rest of LATAM
10.5.5.1. Market Revenue and Forecast, by Component (2017-2030)
10.5.5.2. Market Revenue and Forecast, by Application (2017-2030)
10.5.5.3. Market Revenue and Forecast, by End User (2017-2030)
Chapter 11. Company Profiles
11.1. IQVIA
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. IBM
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. PPD, Inc.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Parexel International Corp.
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. PerkinElmer, Inc.
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Icon Plc
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Oracle
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Syneos Health
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Cegedim Health Data
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Medpace
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/39021
Contact Us:
Vision Research Reports
Call: +1 9197 992 333
