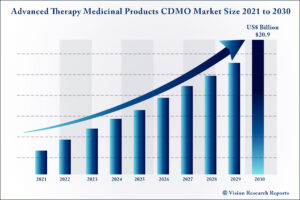
The global Advanced Therapy Medicinal Products CDMO market size is expected to be worth around US$ 20.9 billion by 2030, according to a new report by Vision Research Reports.
The global Advanced Therapy Medicinal Products CDMO market size was valued at US$ 7.29 billion in 2020 and is anticipated to grow at a CAGR of 13.0% during forecast period 2021 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/39014
Table of Contents
Advanced Therapy Medicinal Products CDMO Market Growth Factors
The growing demand for advanced therapy is the key factor fueling the market growth. The growth is attributed to the increasing prevalence of rare and life-threatening diseases, such as metabolic and optical diseases, and rising investment in R&D of advanced therapy medicinal product. Besides, ATMPs such as mesenchymal stem cells (MSCs) are a new treatment effective against the COVID-19 virus.
The growth of the market is credited to the increasing clinical trials of ATMP and the rising awareness and belief among researchers regarding the benefits of advanced therapy. The COVID-19 pandemic has significantly disrupted the cell and gene therapy industry due to the complexity in the manufacturing process.
Advanced Therapy Medicinal Products CDMO Market Report Coverage
| Report Scope | Details |
| Market Size | US$ 20.9 billion by 2030 |
| Growth Rate | CAGR of 13.0% From 2021 to 2030 |
| Base Year | 2021 |
| Forecast Period | 2021 to 2030 |
| Segments Covered | Product, Phase, Indication |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | Celonic; Bio Elpida; CGT Catapult; Rentschler Biopharma SE; AGC Biologics; Catalent; Lonza; WuXi Advanced Therapies; BlueReg; Minaris Regenerative Medicine; Patheon |
By Product Analysis
The gene therapy accounted for the largest revenue share of over 51.0% in 2020. The rapid growth of the segment is attributed to the advancements in therapy as the treatment can alter and improve the genetics or specifically modify the targeted therapeutic treatment.
In the last few years, gene therapy has observed lucrative growth due to the efficiency in invading cells and initiating the genetic materials. The most common method used in gene therapy is recombinant DNA technology.
By Indication Analysis
Oncology accounted for the largest revenue share of over 46.0% in 2020. The growth of the segment is attributed to the increasing prevalence of cancer and chronic diseases owing to the rising geriatric population. Oncology is a branch of medicines, which diagnoses and treats cancer.
According to the WHO, cancer is the second-largest leading cause of death globally and nearly 10 million people died due to cancer in 2020. Most of the cancer cases have been found in undeveloped nations and it mainly affects the low- or middle-income countries due to the lack of a proper medical system.
By Phase Analysis
Phase III accounted for the largest revenue share of over 51.0% in 2020. The growth of the segment is owing to the fact that phase III trials involve a large number of patients, and this phase is also the most extensive study period as it includes a comparison of the efficiency and safety of the new drug.
Phase I held the second-largest revenue share in 2020 owing to the growing R&D activities. With the advent of the Covid-19 pandemic and the use of ATMPs in the treatment of infectious diseases, the segment is expected to witness lucrative growth over the next 2-3 years.
By Regional Analysis
North America held the largest revenue share of over 45.0% in 2020 owing to the growing awareness regarding advanced therapy and rising outsourcing activities.
The region has a favorable regulatory environment as the FDA has approved a few gene therapy products for sale in the U.S. such as ALLOCORD and ABECMA. According to the reports by American Biopharmaceutical Companies.
Read also @ Orthopedic Splints Market to Reach US$ 6.76 Bn by 2030
Major Key Players Covered in The Advanced Therapy Medicinal Products CDMO Market Report include
- Celonic
- Bio Elpida
- CGT Catapult
- Rentschler Biopharma SE
- AGC Biologics
- Catalent
- Lonza
- WuXi Advanced Therapies
- BlueReg
- Minaris Regenerative Medicine
- Patheon
Advanced Therapy Medicinal Products CDMO Market Segmentation
- By Product
- Gene Therapy
- Cell Therapy
- Tissue Engineered
- Others (Combined ATMPs, for example biodegradable matric or scaffold)
- By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
- By Indication
- Oncology
- Cardiology
- Central Nervous System
- Musculoskeletal
- Infectious Disease
- Dermatology
- Endocrine, Metabolic, Genetic
- Immunology & Inflammation
- Ophthalmology
- Haematology
- Gastroenterology
- Others
- Regional
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Chile
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Israel
- Egypt
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Advanced Therapy Medicinal Products CDMO Market, By Product
7.1. Advanced Therapy Medicinal Products CDMO Market, by Product, 2021-2030
7.1.1. Gene Therapy
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Cell Therapy
7.1.2.1. Market Revenue and Forecast (2017-2030)
7.1.3. Tissue Engineered
7.1.3.1. Market Revenue and Forecast (2017-2030)
7.1.4. Others (Combined ATMPs, for example biodegradable matric or scaffold)
7.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Advanced Therapy Medicinal Products CDMO Market, By Phase
8.1. Advanced Therapy Medicinal Products CDMO Market, by Phase, 2021-2030
8.1.1. Phase I
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Phase II
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Phase III
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Phase IV
8.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Advanced Therapy Medicinal Products CDMO Market, By Indication
9.1. Advanced Therapy Medicinal Products CDMO Market, by Indication, 2021-2030
9.1.1. Oncology
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Cardiology
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Central Nervous System
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Musculoskeletal
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. Infectious Disease
9.1.5.1. Market Revenue and Forecast (2017-2030)
9.1.6. Dermatology
9.1.6.1. Market Revenue and Forecast (2017-2030)
9.1.7. Endocrine, Metabolic, Genetic
9.1.7.1. Market Revenue and Forecast (2017-2030)
9.1.8. Immunology & Inflammation
9.1.8.1. Market Revenue and Forecast (2017-2030)
9.1.9. Ophthalmology
9.1.9.1. Market Revenue and Forecast (2017-2030)
9.1.10. Haematology
9.1.10.1. Market Revenue and Forecast (2017-2030)
9.1.11. Gastroenterology
9.1.11.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Advanced Therapy Medicinal Products CDMO Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Product (2017-2030)
10.1.2. Market Revenue and Forecast, by Phase (2017-2030)
10.1.3. Market Revenue and Forecast, by Indication (2017-2030)
10.1.4. U.S.
10.1.4.1. Market Revenue and Forecast, by Product (2017-2030)
10.1.4.2. Market Revenue and Forecast, by Phase (2017-2030)
10.1.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.1.5. Rest of North America
10.1.5.1. Market Revenue and Forecast, by Product (2017-2030)
10.1.5.2. Market Revenue and Forecast, by Phase (2017-2030)
10.1.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Product (2017-2030)
10.2.2. Market Revenue and Forecast, by Phase (2017-2030)
10.2.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2.4. UK
10.2.4.1. Market Revenue and Forecast, by Product (2017-2030)
10.2.4.2. Market Revenue and Forecast, by Phase (2017-2030)
10.2.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2.5. Germany
10.2.5.1. Market Revenue and Forecast, by Product (2017-2030)
10.2.5.2. Market Revenue and Forecast, by Phase (2017-2030)
10.2.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2.6. France
10.2.6.1. Market Revenue and Forecast, by Product (2017-2030)
10.2.6.2. Market Revenue and Forecast, by Phase (2017-2030)
10.2.6.3. Market Revenue and Forecast, by Indication (2017-2030)
10.2.7. Rest of Europe
10.2.7.1. Market Revenue and Forecast, by Product (2017-2030)
10.2.7.2. Market Revenue and Forecast, by Phase (2017-2030)
10.2.7.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Product (2017-2030)
10.3.2. Market Revenue and Forecast, by Phase (2017-2030)
10.3.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3.4. India
10.3.4.1. Market Revenue and Forecast, by Product (2017-2030)
10.3.4.2. Market Revenue and Forecast, by Phase (2017-2030)
10.3.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3.5. China
10.3.5.1. Market Revenue and Forecast, by Product (2017-2030)
10.3.5.2. Market Revenue and Forecast, by Phase (2017-2030)
10.3.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3.6. Japan
10.3.6.1. Market Revenue and Forecast, by Product (2017-2030)
10.3.6.2. Market Revenue and Forecast, by Phase (2017-2030)
10.3.6.3. Market Revenue and Forecast, by Indication (2017-2030)
10.3.7. Rest of APAC
10.3.7.1. Market Revenue and Forecast, by Product (2017-2030)
10.3.7.2. Market Revenue and Forecast, by Phase (2017-2030)
10.3.7.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Product (2017-2030)
10.4.2. Market Revenue and Forecast, by Phase (2017-2030)
10.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4.4. GCC
10.4.4.1. Market Revenue and Forecast, by Product (2017-2030)
10.4.4.2. Market Revenue and Forecast, by Phase (2017-2030)
10.4.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4.5. North Africa
10.4.5.1. Market Revenue and Forecast, by Product (2017-2030)
10.4.5.2. Market Revenue and Forecast, by Phase (2017-2030)
10.4.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4.6. South Africa
10.4.6.1. Market Revenue and Forecast, by Product (2017-2030)
10.4.6.2. Market Revenue and Forecast, by Phase (2017-2030)
10.4.6.3. Market Revenue and Forecast, by Indication (2017-2030)
10.4.7. Rest of MEA
10.4.7.1. Market Revenue and Forecast, by Product (2017-2030)
10.4.7.2. Market Revenue and Forecast, by Phase (2017-2030)
10.4.7.3. Market Revenue and Forecast, by Indication (2017-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Product (2017-2030)
10.5.2. Market Revenue and Forecast, by Phase (2017-2030)
10.5.3. Market Revenue and Forecast, by Indication (2017-2030)
10.5.4. Brazil
10.5.4.1. Market Revenue and Forecast, by Product (2017-2030)
10.5.4.2. Market Revenue and Forecast, by Phase (2017-2030)
10.5.4.3. Market Revenue and Forecast, by Indication (2017-2030)
10.5.5. Rest of LATAM
10.5.5.1. Market Revenue and Forecast, by Product (2017-2030)
10.5.5.2. Market Revenue and Forecast, by Phase (2017-2030)
10.5.5.3. Market Revenue and Forecast, by Indication (2017-2030)
Chapter 11. Company Profiles
11.1. Company 1
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Company 2
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Company 3
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Company 4
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. Company 5
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Company 6
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Company 7
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Company 8
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Company 9
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Company 10
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/39014
Contact Us:
Vision Research Reports
Call: +1 9197 992 333
