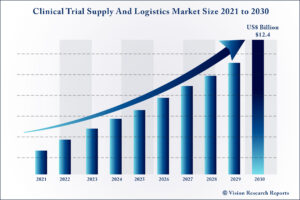
The global clinical trial supply and logistics market size is expected to be worth around US$ 12.4 billion by 2030, according to a new report by Vision Research Reports.
The global clinical trial supply and logistics market size was valued at US$ 5.1 billion in 2020 and is anticipated to grow at a CAGR of 9.1% during forecast period 2021 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/38699
Table of Contents
Clinical Trial Supply And Logistics Market Growth Factors
The market growth is attributed to the increasing R&D expenditure by pharmaceutical and biopharmaceutical firms and the rising number of clinical trials conducted globally. Furthermore, the market is mostly driven by technological advancements in the supply chain. The growing complexity in clinical studies and increased competition among players are factors responsible for the adoption of new technologies in supply chain management.
clinical trials have increased due to the rising demand for the Covid-19 vaccine. According to the WHO, more than 80 vaccines are currently being studied in clinical trials, with over 180 in the pre-clinical stage. There would be a challenge for logistics related to timelines and access to a large population.
Clinical Trial Supply And Logistics Market Report Coverage
| Report Scope | Details |
| Market Size | USD 12.4 billion by 2030 |
| Growth Rate | CAGR of 9.1% From 2021 to 2030 |
| Base Year | 2021 |
| Forecast Period | 2021 to 2030 |
| Segments Covered | Service, Phase, End-user, Therapeutic area |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | Thermo Fisher Scientific (U.S.) (Patheon); Catalent, Inc. (U.S.); Parexel (U.S.); Almac Group; Marken; Piramal Pharma Solutions; UDG Healthcare; DHL; FedEx; Movianto; Packaging Coordinators Inc. |
Clinical Trial Supply And Logistics Market By Service Analysis
The logistics and distribution segment led the market and accounted for a revenue share of over 20.0% in 2020. The segment is also anticipated to register the fastest growth rate throughout the forecast period. This is due to the globalization of clinical trials, an increase in the number of trials involving temperature-sensitive products, and the highly regulated structure of the industry.
Technological developments are positively impacting the global market, both on the inbound and outbound sides. As additional Point of Care (POC) diagnostic tools are developed, there would be a trend toward performing more and more local analyses and transferring less blood, tissue, urine, and other samples to a central site for examination in the future.
Advances in smart packaging solutions, such as active and passive thermo-regulated products, ensure that shipments stay within suitable temperature ranges while en-route and in storage, protecting the contents of the shipment
Clinical Trial Supply And Logistics Market By Phase Analysis
The phase III segment held the largest revenue share of over 40.0% in 2020. This is primarily due to a rise in the number of patients enrolled in phase III trials. Investigators in phase III studies compare the novel treatment’s safety and efficacy against the current standard of care.
Phase II clinical trials had the highest number of projects in 2020 and this trend is expected to continue owing to increasing investments in R&D by industry and non-industry sponsors.
Clinical Trial Supply And Logistics Market By Therapeutic Area Analysis
The cardiovascular diseases segment led the largest revenue share of over 25.0% in 2020. This is due to the large and growing number of cardiovascular research studies and the growing number of companies focused on bringing innovative drugs to the market.
The oncology segment held the second-largest share in 2020. This is due to the high prevalence of cancer. In 2018, cancer was the second biggest cause of death around the world, accounting for 9.6 million fatalities, or one in every six deaths.
Clinical Trial Supply And Logistics Market By End-user Analysis
Based on end-user, the pharmaceuticals segment led the market and accounted for over 40.0% share in 2020. This is due to the increased number of clinical studies conducted by pharmaceutical companies and R&D investments by these companies. Besides, the globalization of clinical trials is supporting market growth.
The biologicals segment is projected to expand at the fastest growth rate of 7.3% over the forecast period. This may be largely attributed to the increasing demand for biological products, such as cell and gene therapies, and vaccines and the growing investments in product development
Clinical Trial Supply And Logistics Market By Regional Analysis
North America held the global market with a share of over 35.0% in 2020. An increase in the number of clinical trials conducted by pharmaceutical companies drives the market in the region. According to ClinicalTrials.gov, the U.S. performs the highest number of clinical trial operations per year.
Asia Pacific is anticipated to register the fastest growth rate of7.8% throughout the forecast period. The region has access to a large and diversified patient pool, cheaper recruitment costs, and favorable policies that make Asia Pacific desirable for clinical studies.
Clinical Trial Supply And Logistics Market Key Players
- Piramal Pharma Solutions
- UDG Healthcare
- DHL
- FedEx
- Movianto
- Packaging Coordinators Inc.
- Thermo Fisher Scientific (Patheon)
- Catalent, Inc.
- Parexel
- Almac Group
- Marken
Read also @ Transportation Management Systems Market Size, Trends & Growth [2030]
Clinical Trial Supply And Logistics Market Segmentation
- Service
- Logistics & Distribution
- Storage & Retention
- Packaging, Labeling, and Blinding
- Manufacturing
- Comparator Sourcing
- Other Services (Solutions, Ancillary Supply)
- Phase
- Phase I
- Phase II
- Phase III
- Phase IV
- End-user
- Pharmaceuticals
- Biologicals
- Medical Device
- Therapeutic Area
- Oncology
- Cardiovascular Diseases
- Respiratory Diseases
- CNS and Mental Disorders
- Others
- Regional
- North America
- U.S
- Canada
- Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Asia Pacific
- Japan
- China
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Chile
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Iran
- Israel
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Clinical Trial Supply And Logistics Market Snapshot
Chapter 4. Clinical Trial Supply And Logistics Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and End-user Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Clinical Trial Supply And Logistics Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Clinical Trial Supply And Logistics Market, By Service
7.1. Clinical Trial Supply And Logistics Market, by Service, 2021-2030
7.1.1. Logistics & Distribution
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Storage & Retention
7.1.2.1. Market Revenue and Forecast (2017-2030)
7.1.3. Packaging, Labeling, and Blinding
7.1.3.1. Market Revenue and Forecast (2017-2030)
7.1.4. Manufacturing
7.1.4.1. Market Revenue and Forecast (2017-2030)
7.1.5. Comparator Sourcing
7.1.5.1. Market Revenue and Forecast (2017-2030)
7.1.6. Other Services (Solutions, Ancillary Supply)
7.1.6.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Clinical Trial Supply And Logistics Market, By Phase
8.1. Clinical Trial Supply And Logistics Market, by Phase, 2021-2030
8.1.1. Phase I
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Phase II
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Phase III
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Phase IV
8.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Clinical Trial Supply And Logistics Market, By End-user
9.1. Clinical Trial Supply And Logistics Market, by End-user, 2021-2030
9.1.1. Pharmaceuticals
9.1.1.1. Clinical Trial Supply And Logistics Market Revenue and Forecast (2017-2030)
9.1.2. Biologicals
9.1.2.1. Clinical Trial Supply And Logistics Market Revenue and Forecast (2017-2030)
9.1.3. Medical Device
9.1.3.1. Clinical Trial Supply And Logistics Market Revenue and Forecast (2017-2030)
Chapter 10. Global Clinical Trial Supply And Logistics Market, By Therapeutic Area
10.1. Clinical Trial Supply And Logistics Market, by Therapeutic Area, 2021-2030
10.1.1. Oncology
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Cardiovascular Diseases
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Respiratory Diseases
10.1.3.1. Market Revenue and Forecast (2017-2030)
10.1.4. CNS and Mental Disorders
10.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Clinical Trial Supply And Logistics Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Service (2017-2030)
11.1.2. Market Revenue and Forecast, by Phase (2017-2030)
11.1.3. Market Revenue and Forecast, by End-user (2017-2030)
11.1.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.1.5. U.S.
11.1.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Phase (2017-2030)
11.1.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.1.5.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.1.6. Rest of North America
11.1.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.1.6.2. Market Revenue and Forecast, by Phase (2017-2030)
11.1.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.1.6.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.2. Market Revenue and Forecast, by Phase (2017-2030)
11.2.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.5. UK
11.2.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Phase (2017-2030)
11.2.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.5.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.6. Germany
11.2.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Phase (2017-2030)
11.2.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.6.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.7. France
11.2.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Phase (2017-2030)
11.2.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.7.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.8. Rest of Europe
11.2.8.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.8.2. Market Revenue and Forecast, by Phase (2017-2030)
11.2.8.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.8.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.2. Market Revenue and Forecast, by Phase (2017-2030)
11.3.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.5. India
11.3.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Phase (2017-2030)
11.3.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.5.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.6. China
11.3.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Phase (2017-2030)
11.3.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.6.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.7. Japan
11.3.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Phase (2017-2030)
11.3.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.7.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.8. Rest of APAC
11.3.8.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.8.2. Market Revenue and Forecast, by Phase (2017-2030)
11.3.8.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.8.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.2. Market Revenue and Forecast, by Phase (2017-2030)
11.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.5. GCC
11.4.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Phase (2017-2030)
11.4.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.5.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.6. North Africa
11.4.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Phase (2017-2030)
11.4.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.6.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.7. South Africa
11.4.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Phase (2017-2030)
11.4.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.7.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.8. Rest of MEA
11.4.8.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.8.2. Market Revenue and Forecast, by Phase (2017-2030)
11.4.8.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.8.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.5.2. Market Revenue and Forecast, by Phase (2017-2030)
11.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.5.5. Brazil
11.5.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Phase (2017-2030)
11.5.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5.5.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.5.6. Rest of LATAM
11.5.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.5.6.2. Market Revenue and Forecast, by Phase (2017-2030)
11.5.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5.6.4. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
Chapter 12. Company Profiles
12.1. Piramal Pharma Solutions
12.1.1. Company Overview
12.1.2. Service Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. UDG Healthcare
12.2.1. Company Overview
12.2.2. Service Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. DHL
12.3.1. Company Overview
12.3.2. Service Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. FedEx
12.4.1. Company Overview
12.4.2. Service Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Movianto
12.5.1. Company Overview
12.5.2. Service Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Packaging Coordinators Inc.
12.6.1. Company Overview
12.6.2. Service Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Thermo Fisher Scientific (Patheon)
12.7.1. Company Overview
12.7.2. Service Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Catalent, Inc.
12.8.1. Company Overview
12.8.2. Service Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Parexel
12.9.1. Company Overview
12.9.2. Service Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Almac Group
12.10.1. Company Overview
12.10.2. Service Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/38699
Contact Us:
Vision Research Reports
Call: +1 9197 992 333