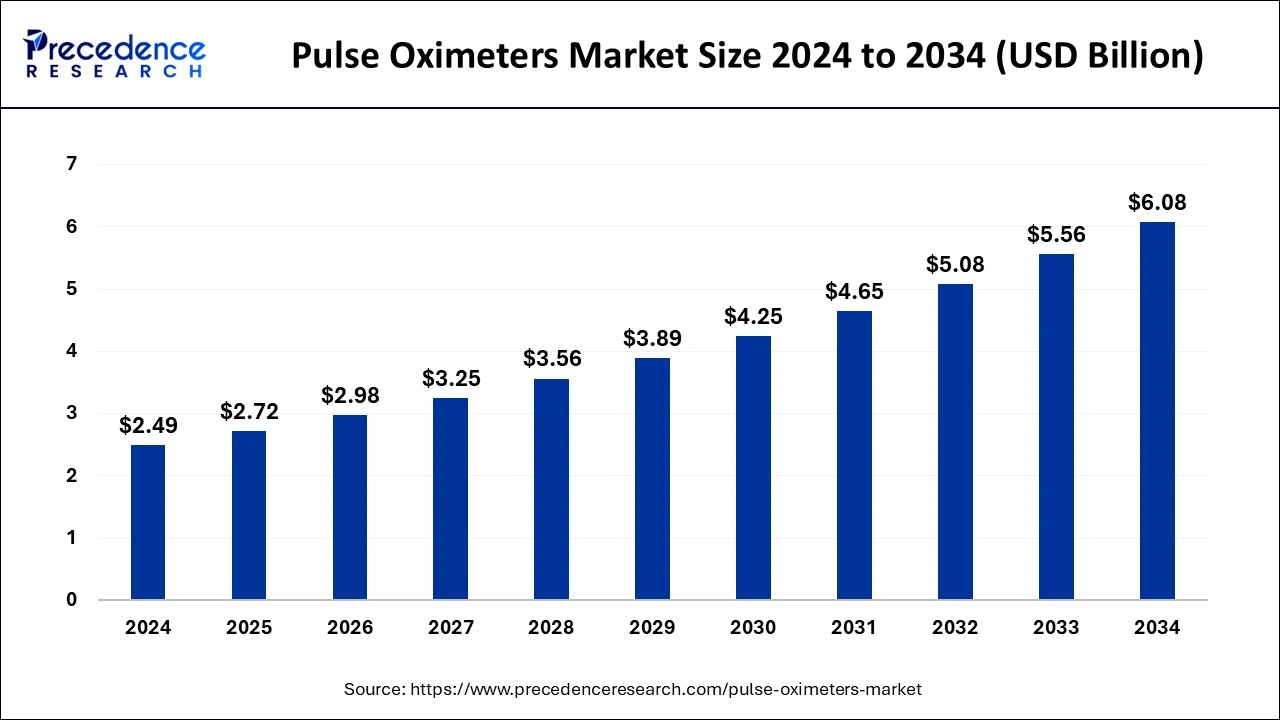
The pulse oximeters market size was estimated at USD 2.49 billion in 2024, and it is expected to be reach around USD 6.08 billion by 2034 with a CAGR of 9.33%.
Get Sample Copy of Report @ https://www.precedenceresearch.com/sample/1036
Key Insights
- In 2024, North America accounted for 49% of the market share, the highest among all regions.
- The hospitals segment dominated the market by end-use in 2024.
- The home healthcare segment is projected to grow at the fastest CAGR during the forecast period.
- Among product types, tabletop oximeters held the largest market share in 2024.
The Growing Influence of AI in Pulse Oximeters Market
AI technology is playing a crucial role in optimizing pulse oximeters by refining data accuracy and automating analysis. By leveraging machine learning, modern pulse oximeters can adapt to various patient conditions, reducing inaccuracies often seen in traditional models. AI integration is also facilitating better connectivity with wearable technology and healthcare ecosystems, making real-time monitoring and early intervention more efficient. The future of pulse oximeters is likely to see even greater advancements as AI capabilities expand.
Market Drivers
The growing awareness of oxygen saturation monitoring in critical care and home healthcare settings is propelling the pulse oximeters market forward. The demand for continuous and remote patient monitoring is rising, particularly among patients with chronic respiratory diseases.
Additionally, technological advancements such as AI integration and Bluetooth-enabled devices are improving the efficiency and convenience of pulse oximeters, making them more appealing to both healthcare providers and consumers.
Opportunities
The increasing demand for wearable and smart health devices presents a major growth opportunity for pulse oximeter manufacturers. Innovations in sensor technology and cloud-based monitoring systems enable better connectivity and data analysis, enhancing healthcare efficiency.
The growing trend of self-monitoring and preventive healthcare is also driving market expansion, as individuals are taking a proactive approach to managing their health.
Challenges
One of the significant challenges in the pulse oximeters market is the concern over inaccurate readings due to external factors such as skin pigmentation, nail polish, and ambient lighting. Additionally, counterfeit products and unregulated manufacturers present risks to consumer safety.
The high cost of advanced pulse oximeters may hinder their accessibility in low-income regions, limiting overall market growth.
Regional Insights
North America continues to lead the global pulse oximeters market, fueled by technological innovations and strong healthcare infrastructure. Europe follows with growing demand for home healthcare devices and increased focus on chronic disease management.
The Asia-Pacific region is expected to grow rapidly, driven by rising healthcare awareness and increasing investments in medical technology. Latin America and the Middle East & Africa are also witnessing gradual market growth due to improving healthcare access and rising health consciousness.
Pulse Oximeters Market Companies
- Smiths Group plc.
- Halmaplc
- Koninklijke Philips N.V.
- Medtronic plc
- Nihon Kohden Corporation
- Masimo Corporation
- Contec Medical Systems Co., Ltd.
- Omron healthcare, Inc.
- General Electric Company
- Nonin Medical, Inc.
Leadership Announcement
Prevounce Health comes as one of the leading providers of software for remote care management by announcing its first blood oxygen device under the venture of remote patient monitoring (RPM): Pylo OX1-LTE. According to Daniel Tashnek, founder and Chief Executive Officer of Prevounce, this device was an exciting addition to the Pylo family.
Even as the device delivers actionable data to clinicians for empowering care, it will be allowed to be used much more easily by patients. This will ensure increased involvement and participation in RPM programs while reducing both clinician and technical support work needed to scale up remote monitoring programs.
Recent Developements
- In January 2025, the United States Food and Drug Administration released guidelines for manufacturers regarding the calibration of their devices to better read pulse oxygen readings for people of color, by ensuring devices are tested across a variety of skin tones. The new guidelines ask companies to mandate the collection of 3,000 data points rather than 200. Studies conducted using these meters will also need to be composed of a minimum of 10 people, with 150 being the recommended sample size.
- In August 2024, Prevounce Health, a leading medical device provider, launched a remote patient-monitoring blood oxygen measurement device called Pylo OX1-LTE. The device is cellular connected, offering secure data collection, reliability and improved connectivity. The device is cost-effective, making it easier for organizations to invest in growing their remote patient monitoring programs.
Segments Covered in the Report
By Product Type
- Handheld Oximeters
- Fingertip Oximeters
- TabletopOximeters
By End-user
- Ambulatory Surgical Centers
- Hospitals
- Home Healthcares
By Regional Outlook
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/