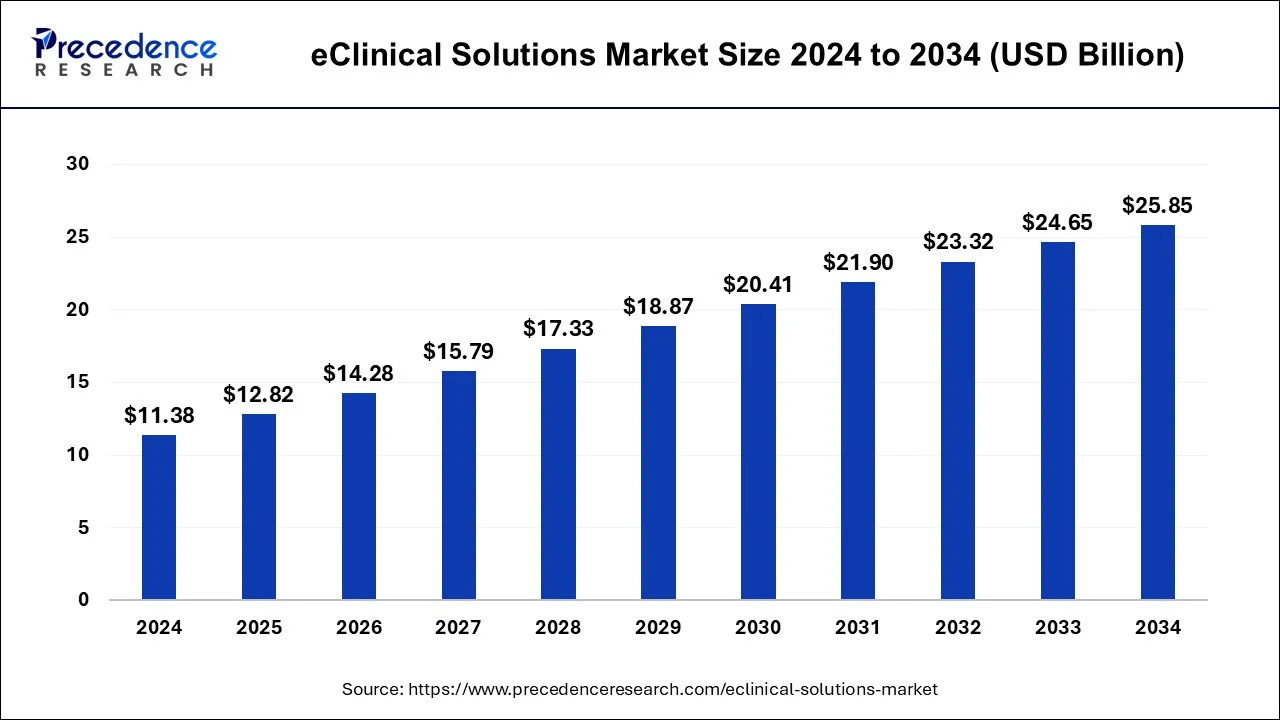
The global eClinical solutions market size reached USD 11.38 billion in 2024 and is expected to surpass around USD 25.85 billion by 2034 with a solid CAGR of 8.10%.
Get Sample Copy of Report@ https://www.precedenceresearch.com/sample/1092
Key Insights
- North America dominated the eClinical solutions market in 2024, holding the largest market share of 47.93%.
- The web-hosted segment led the market by delivery mode, securing a revenue share of over 50.49% in 2024.
- The phase III segment emerged as the top contributor in the development phase category, accounting for more than 43.71% of the revenue share in 2024.
- The CROs segment held the largest share in the eClinical solutions market, contributing over 39.38% of the total revenue in 2024.
Market Dynamics
Market Drivers
The increasing complexity of clinical trials and the growing demand for efficient data management are key drivers fueling the growth of the eClinical solutions market. The adoption of digital technologies in clinical research, coupled with the rising need for remote monitoring, has significantly accelerated market expansion.
Regulatory agencies worldwide are encouraging the use of electronic data capture (EDC) and other eClinical tools to improve trial efficiency and ensure data accuracy. Additionally, the growing prevalence of chronic diseases and the rise in clinical trial activities further contribute to market growth.
Opportunities
The integration of artificial intelligence and machine learning in eClinical solutions presents vast opportunities for market expansion. These technologies enable better predictive analytics, improving trial design and patient recruitment processes.
The growing adoption of decentralized clinical trials (DCTs) and the expansion of cloud-based solutions also create new prospects for market players. Moreover, emerging economies investing in digital healthcare infrastructure offer untapped potential for eClinical solution providers.
Challenges
Despite the rapid advancements, the eClinical solutions market faces challenges related to data security and compliance with stringent regulatory requirements. The interoperability issues between different eClinical platforms and legacy systems hinder seamless data exchange, affecting trial efficiency.
Additionally, the high initial implementation costs and lack of skilled professionals to manage complex eClinical solutions present obstacles to widespread adoption.
Regional Insights
North America leads the eClinical solutions market, driven by strong regulatory support, advanced healthcare infrastructure, and the presence of major industry players. Europe follows closely, with increasing investments in clinical research and stringent data protection regulations promoting digital transformation.
The Asia-Pacific region is expected to witness significant growth due to rising clinical trial activities and advancements in healthcare IT. Meanwhile, Latin America and the Middle East & Africa are gradually adopting eClinical solutions, offering growth opportunities in the long run.
eClinical Solutions Market Companie
- PAREXEL International
- Oracle Corp.
- Bioclinica
- Medidata Solution
- DATATRAK
- ERT Clinical
- CRF Health
- eClinicalWorks
- OmniComm Systems
- IBM Watson Health
- eClinical Solutions
Segments Covered in the Report
This research study comprises complete assessment of the market by means of far-reaching qualitative and quantitative perceptions, and predictions regarding the market. This report delivers classification of marketplace into impending and niche sectors. Further, this research study calculates market size and its development drift at global, regional, and country from 2020 to 2032. This report contains market breakdown and its revenue estimation by classifying it on the basis of product, development phase, delivery mode, end-use, and region:
By Solution
- Randomization and Trial Supply Management
- Clinical Data Management System
- Clinical Trial Management System
- Electronic Clinical Outcome Assessment
- Electronic Trial Master Files
- Electronic Data Capture
- Others
By Development Phase
- Phase IV
- Phase III
- Phase II
- Phase I
By Delivery Mode
- Licensed Enterprise
- Web-hosted
- Cloud-based
By End-Use
- CROs
- Hospitals
- Academic Institutes
- Medical Device Manufacturers
- Pharma & Biotech Organizations
By Regional Outlook
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/