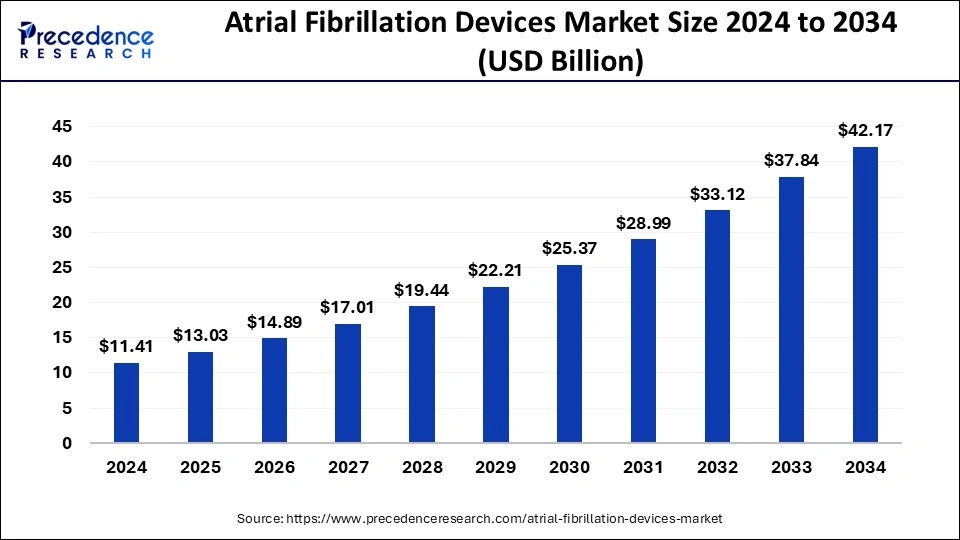
The global atrial fibrillation devices market size was estimated at USD 9.99 billion in 2023 and is projected to reach around USD 37.84 billion by 2033, growing at a CAGR of 14.25% from 2024 to 2033.
Key Points
- North America dominated the market share of 40% in 2023.
- By product, the EP ablation catheters segment dominated the atrial fibrillation devices market in 2023.
- By end-use, the hospitals segment dominated the market in 2023.
The atrial fibrillation (AF) devices market encompasses various medical devices used in the diagnosis, treatment, and management of atrial fibrillation, a common heart rhythm disorder characterized by irregular and often rapid heartbeats. These devices play a crucial role in restoring normal heart rhythm, reducing symptoms, preventing complications, and improving the quality of life for patients with AF. The market includes a wide range of devices such as catheter ablation systems, implantable cardioverter defibrillators (ICDs), pacemakers, cardiac monitors, and other emerging technologies aimed at addressing the growing prevalence of AF globally.
Get a Sample: https://www.precedenceresearch.com/sample/3950
Growth Factors Driving the Atrial Fibrillation Devices Market
Several factors contribute to the growth of the atrial fibrillation devices market. One significant factor is the increasing prevalence of atrial fibrillation worldwide, driven by aging populations, rising incidences of lifestyle-related risk factors such as obesity, hypertension, and diabetes, and advancements in diagnostic techniques leading to better detection rates. Additionally, the growing adoption of minimally invasive procedures such as catheter ablation for the treatment of AF is fueling the demand for AF devices. Moreover, technological advancements in device design, materials, and software algorithms are enhancing the efficacy, safety, and patient outcomes associated with AF device therapies, further driving market growth.
Region Insights into the Atrial Fibrillation Devices Market
The atrial fibrillation devices market exhibits regional variations influenced by factors such as healthcare infrastructure, regulatory environment, prevalence of AF, reimbursement policies, and adoption of advanced medical technologies. North America dominates the market, driven by high healthcare expenditure, a large patient pool, well-established healthcare infrastructure, and a favorable reimbursement scenario. Europe follows closely, characterized by increasing adoption of minimally invasive procedures and growing awareness about AF management. The Asia-Pacific region is poised for significant growth, fueled by improving healthcare access, rising disposable incomes, and a surge in AF cases due to demographic and lifestyle changes.
Atrial Fibrillation Devices Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 14.25% |
| Global Market Size in 2023 | USD 9.99 Billion |
| Global Market Size by 2033 | USD 37.84 Billion |
| U.S. Market Size in 2023 | USD 3 Billion |
| U.S. Market Size by 2033 | USD 11.35 Billion |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Product and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Atrial Fibrillation Devices Market Dynamics
Drivers of Growth in the Atrial Fibrillation Devices Market
Several drivers propel the growth of the atrial fibrillation devices market. One key driver is the growing demand for effective and minimally invasive treatment options for AF, spurring the development and adoption of innovative devices. Moreover, the expanding elderly population, who are at higher risk of developing AF, is driving market growth. Additionally, initiatives by healthcare organizations and government bodies to raise awareness about AF, improve diagnosis rates, and enhance treatment options are further driving market expansion. Furthermore, strategic collaborations, partnerships, and mergers among key market players are facilitating the development and commercialization of advanced AF devices, contributing to market growth.
Opportunities in the Atrial Fibrillation Devices Market
The atrial fibrillation devices market presents numerous opportunities for growth and innovation. One significant opportunity lies in the development of next-generation AF devices with improved efficacy, safety, and patient comfort. Additionally, expanding market penetration in emerging economies presents untapped opportunities for manufacturers to address the unmet medical needs of a growing AF patient population. Furthermore, advancements in digital health technologies, such as remote monitoring and telemedicine solutions, offer opportunities to enhance patient care, adherence to treatment, and clinical outcomes in AF management. Moreover, the integration of artificial intelligence and machine learning algorithms into AF devices holds promise for personalized treatment approaches and predictive analytics, opening new avenues for market expansion.
Challenges Facing the Atrial Fibrillation Devices Market
Despite the promising growth prospects, the atrial fibrillation devices market faces several challenges. One major challenge is the high cost associated with AF devices and procedures, limiting access to advanced treatments, particularly in developing regions with constrained healthcare budgets. Moreover, regulatory complexities and stringent approval processes pose challenges for market entry and product commercialization. Additionally, the limited clinical evidence supporting the long-term efficacy and cost-effectiveness of certain AF devices may hinder widespread adoption and reimbursement coverage. Furthermore, the presence of alternative treatment modalities such as pharmacological therapy and surgical interventions poses competitive challenges to device-based approaches for AF management. Addressing these challenges will require collaborative efforts among industry stakeholders, regulatory bodies, healthcare providers, and patient advocacy groups to drive innovation, improve access, and optimize patient outcomes in the management of atrial fibrillation.
Read Also: Cardiac Arrhythmia Monitoring Devices Market Size, Share, Report 2033
Recent Developments
- In November 2023, Medtronic introduced a heart implant to reduce the lifetime risk of stroke in patients with atrial fibrillation and improve the quality of life for patients undergoing open cardiac surgery.
- In January 2022, AliveCor, Inc. and Voluntis, a leading Aptar Pharma firm in digital therapies, have partnered to provide advanced management of atrial fibrillation for cancer patients.
Atrial Fibrillation Devices Market Companies
- Abbott Laboratories
- Johnson & Johnson
- Atricure Inc
- Microport Scientific Corporation
- Boston Scientific Corporation
- St. Jude Medical, Inc
- Medtronic Plc
- Koninklijke Philips N.V.
- Siemens AG
Segments Covered in the Report
By Product
- EP Ablation Catheters
- EP Diagnostic Catheters
- Mapping and Recording Systems
- Cardiac Monitors or Implantable Loop Recorder
- Access Devices
- Intracardiac Echocardiography (ICE)
- Left Atrial Appendage (LAA) Closure Devices
By End-use
- Hospitals
- Cardiac Centers
- Ambulatory Surgical Centers
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/