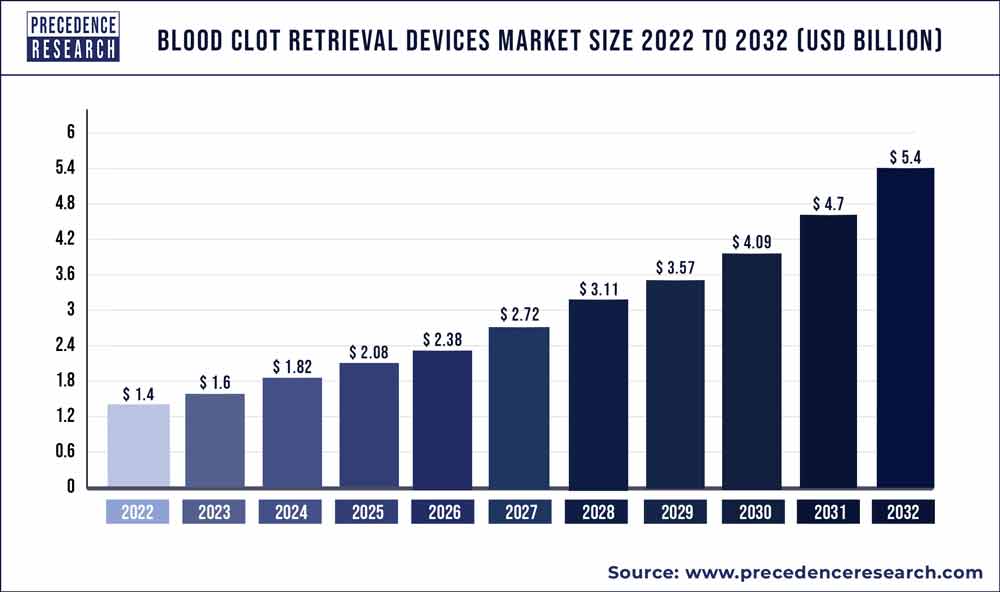
Precedence Research a new report on blood clot retrieval devices market size, share, growth, industry trends, and forecast 2030, covering various industry elements and growth trends helpful for predicting the market’s future. The global blood clot retrieval devices market size was valued at US$ 1.3 billion in 2021. The global blood clot retrieval devices market is projected to reach US$ 4.5 billion by 2030, registering a compound annual growth rate (CAGR) of 14.79% during the forecast period from 2022 to 2030.
Download Free Sample Copy with TOC@ https://www.precedenceresearch.com/sample/1808
The global blood clot retrieval devices market report offers various segments analysis by size, trends, growth factors, opportunities and key country outlook to 2030. The report offers a detailed analysis and information as per blood clot retrieval devices, market segments helping our readers to get a comprehensive overview of the global market. Several players are planning to focus on developing cost-effective products or services, aiming to maintain a strong foothold in the market.
Blood Clot Retrieval Devices Market Report Scope
| Report Coverage | Details |
| Market Size by 2030 | USD 4.5 Billion |
| Growth Rate from 2022 to 2030 | CAGR of 14.79% |
| Largest Market | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Stroke, Device, Application, End User, Geography |
The company profiling of leading market players is included this report with Porter’s five forces analysis and Value Chain analysis. Further, the strategies exercised by the companies for expansion of business through mergers, acquisitions, and other business development measures are discussed in the report. The financial parameters which are assessed include the sales, profits and the overall revenue generated by the key players of Market.
Read Also@ Contract Cleaning Services Market Size to Hit Around US$ 533 billion by 2030
Some of the prominent players in the blood clot retrieval devices market include:
- AngioDynamics
- Terumo Co.
- Johnson and Johnson
- ECKOS Co.
- Bayer HealthCare LLC
- Boston Scientific Co.
- Argon Medical Devices
- Medtronic Plc
- Teleflex Incorporated
- Penumbra
Blood Clot Retrieval Devices Market Segmentation
By Stroke
- Ischemic Stroke (blood clot)
- Hemorrhagic Stroke (rupturing of arteries)
- Transient Ischemic Attack
By Device
- Mechanical Embolus Removal Devices
- Penumbra Blood Clot Retrieval Devices
- Stent Retrievers
- Aspiration Device
- Ultrasound Assisted Devices
By Application
- Coronary Arteries
- Peripheral Arteries
- Cerebral Arteries
By End User
- Hospitals
- Diagnostic Centers
- Clinics
- Ambulatory Surgical Centers
Regional Segmentation
- Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
- Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
- North America [United States, Canada, Mexico]
- South America [Brazil, Argentina, Columbia, Chile, Peru]
- Middle East & Africa [GCC, North Africa, South Africa]
Key Questions are
- What are the key factors influencing the blood clot retrieval devices market in each region?
- How much value will the global market generate by the end of the forecast period?
- What will be the CAGR of the global market between 2022 and 2030?
- What would be the Y-o-Y growth trend of the global market between 2022 and 2030?
- What is the future scope and current trends in technologies of the global market?
- What is the revenue of the global market based on segments?
- Which key strategies are used by top players of the global market?
- Which are the leading companies in the global market?
- What are the essential strategies by key stakeholders in the market to expand their geographic presence?
- What are the major advancements witnessed in the global market?
Research Objectives and Research Approach
The comprehensive report on the global blood clot retrieval devices market begins with an overview, followed by the scope and objectives of the study. The report provides detailed explanation of the objectives behind this study and key vendors and distributors operating in the market and regulatory scenario for approval of products. Following this, the report provides detailed explanation of objectives of this study and laid down by accredited agencies in the purview of research in the global blood clot retrieval devices market.
It is followed by market introduction, market dynamics, and an overview of the global market, which includes analysis of market drivers, restraints, and trends pertaining to the global market. Furthermore, Y-o-Y growth analysis with elaborated insights has been provided in order to understand the Y-o-Y growth trend of the global market.
For reading comprehensibility, the report has been compiled in a chapter-wise layout, with each section divided into smaller sections. The report comprises an exhaustive collection of graphs and tables that are appropriately interspersed. Pictorial representation of actual and projected values of key segments is visually appealing to readers. This also allows comparison of the market shares of key segments in the past and at the end of the forecast period.
The report analyzes the global blood clot retrieval devices market in terms of type, application, region and others. Key segments under each criteria are studied at length, and the market share for each of these at the end of 2030 has also been provided. Such valuable insights enable market stakeholders in making informed business decisions for investment in the global market.
Why should you invest in this report?
If you are aiming to enter the global blood clot retrieval devices market, this report is a comprehensive guide that provides crystal clear insights into this niche market. All the major application areas for blood clot retrieval devices are covered in this report and information is given on the important regions of the world where this market is likely to boom during the forecast period of 2022-2030 so that you can plan your strategies to enter this market accordingly.
Besides, through this report, you can have a complete grasp of the level of competition you will be facing in this hugely competitive market and if you are an established player in this market already, this report will help you gauge the strategies that your competitors have adopted to stay as market leaders in this market. For new entrants to this market, the voluminous data provided in this report is invaluable.
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Blood Clot Retrieval Devices Market
5.1. COVID-19 Landscape: Blood Clot Retrieval Devices Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Blood Clot Retrieval Devices Market, By Stroke
8.1. Blood Clot Retrieval Devices Market, by Stroke, 2022-2030
8.1.1. Ischemic Stroke (blood clot)
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Hemorrhagic Stroke (rupturing of arteries)
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Transient Ischemic Attack
8.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Blood Clot Retrieval Devices Market, By Devices
9.1. Blood Clot Retrieval Devices Market, by Devices, 2022-2030
9.1.1. Mechanical Embolus Removal Devices
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Penumbra Blood Clot Retrieval Devices
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Stent Retrievers
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Aspiration Device
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. Ultrasound Assisted Devices
9.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Blood Clot Retrieval Devices Market, By Application
10.1. Blood Clot Retrieval Devices Market, by Application, 2022-2030
10.1.1. Coronary Arteries
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Peripheral Arteries
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Cerebral Arteries
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Blood Clot Retrieval Devices Market, By End User
11.1. Blood Clot Retrieval Devices Market, by End User, 2022-2030
11.1.1. Hospitals
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Diagnostic Centers
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Clinics
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Ambulatory Surgical Centers
11.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Blood Clot Retrieval Devices Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.1.2. Market Revenue and Forecast, by Devices (2017-2030)
12.1.3. Market Revenue and Forecast, by Application (2017-2030)
12.1.4. Market Revenue and Forecast, by End User (2017-2030)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.1.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.1.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.1.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.1.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.1.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.1.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.7.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.7.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.8.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.8.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.7.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.7.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.8.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.8.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.7.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.7.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.8.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.8.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.5.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.5.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.5.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.5.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.5.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.5.6.4. Market Revenue and Forecast, by End User (2017-2030)
Chapter 13. Company Profiles
13.1. AngioDynamics
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Terumo Co.
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Johnson and Johnson
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. ECKOS Co.
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Bayer HealthCare LLC
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Boston Scientific Co.
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Argon Medical Devices
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Medtronic Plc
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Teleflex Incorporated
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Penumbra
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
Buy This Premium Research Report Click Here@ https://www.precedenceresearch.com/checkout/1808
Our Press Release@ https://www.precedenceresearch.com/press-releases
About Us
Precedence Research is a Canada/India based company and one of the leading providers of strategic market insights. We offer executive-level blueprints of markets and solutions beyond flagship surveys. Our repository covers consultation, syndicated market studies, and customized research reports. Through our services we aim at connecting an organization’s goal with lucrative prospects globally.
From gauging investment feasibility to uncovering hidden growth opportunities, our market studies cover in-depth analysis, which also is interspersed with relevant statistics. Recommendation are often enclosed within our reports with the sole intent of enabling organizations achieve mission-critical success.
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Website: https://www.precedenceresearch.com