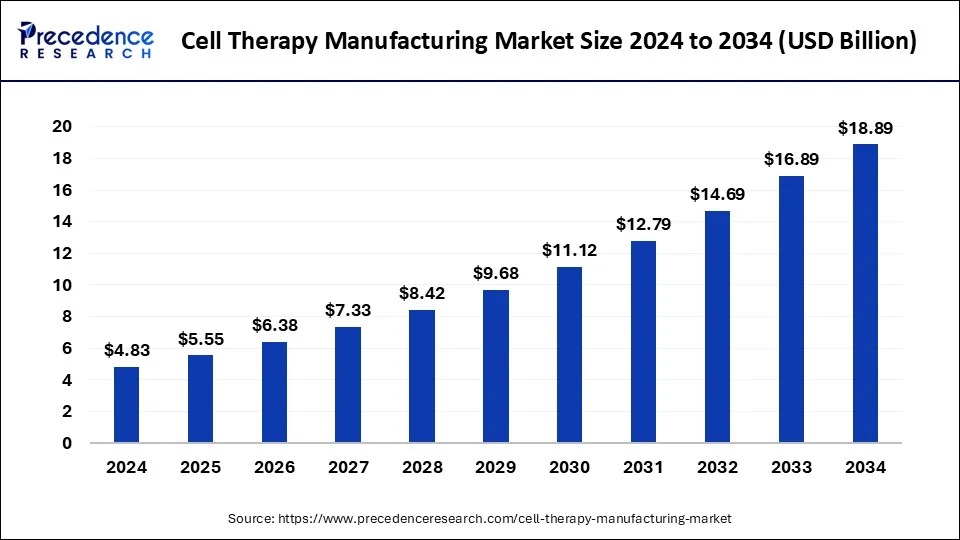
The global cell therapy manufacturing market size reached USD 4.20 billion in 2023 and is predicted to attain around USD 16.89 billion by 2033, growing at a CAGR of 14.93% from 2024 to 2033.
Key Points
- The North America cell therapy manufacturing market size accounted for USD 1.85 billion in 2023 and is expected to attain around USD 7.43 billion by 2033.
- North America led the market with the major revenue share of 44% in 2023.
- Asia Pacific is expected to witness the fastest growth during the forecast period.
- By therapy type, the autologous cell therapy segment has held the biggest revenue share of 59% in 2023.
- By therapy type, the allogenic cell therapy segment is projected to be the fastest-growing segment over the forecast period.
- By technology type, the somatic cell technology segment held the largest share of the market in 2023.
- By technology type, the 3D technology segment is expected to grow at the fastest rate during the projected period.
- By source, the IPSC (induced pluripotent stem cell) segment dominated the market in 2023.
- By source, the bone marrow segment is the second largest segment in the global market.
- By application, the oncology segment has contributed the largest revenue share of 35% in 2023.
- By application, the neurological segment is projected to show fastest growth during the forecast period.
The cell therapy manufacturing market encompasses the processes involved in the production of cellular-based therapies, including cell-based immunotherapies and regenerative medicine. This field represents a revolutionary approach to treating diseases by leveraging the therapeutic potential of cells. The manufacturing of cell therapies involves complex techniques to cultivate, expand, and manipulate cells in a controlled environment, ensuring safety, efficacy, and reproducibility of the final therapeutic product.
Get a Sample: https://www.precedenceresearch.com/sample/4283
Growth Factors
Several key factors are driving the growth of the cell therapy manufacturing market. One significant factor is the increasing prevalence of chronic and degenerative diseases, such as cancer, cardiovascular disorders, and neurological conditions, which are driving demand for innovative treatment options like cell therapies. Moreover, advancements in biotechnology and stem cell research have expanded our understanding of cellular mechanisms, propelling the development of novel cell-based therapies. Additionally, supportive regulatory initiatives and investments in research and development are accelerating the commercialization of cell therapies.
Region Insights
The cell therapy manufacturing market exhibits regional variations influenced by regulatory frameworks, healthcare infrastructure, and research investments. North America holds a prominent position in the market due to a robust biotechnology sector, favorable regulatory policies, and substantial investments in research and development. Europe follows closely, characterized by a strong presence of biopharmaceutical companies and academic institutions engaged in cell therapy development. Emerging economies in Asia-Pacific, such as China and India, are witnessing rapid growth driven by increasing healthcare expenditures and a growing focus on advanced therapies.
Cell Therapy Manufacturing Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 14.93% |
| Cell Therapy Manufacturing Market Size in 2023 | USD 4.20 Billion |
| Cell Therapy Manufacturing Market Size in 2024 | USD 4.83 Billion |
| Cell Therapy Manufacturing Market Size by 2033 | USD 16.89 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Therapy Type, By Technology Type, By Source, and By Application |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Cell Therapy Manufacturing Market Dynamics
Drivers
Several drivers contribute to the expansion of the cell therapy manufacturing market. Technological advancements in cell culture techniques, automation, and bioprocessing equipment are enhancing manufacturing efficiency and scalability. Collaborations between academic institutions, research organizations, and industry players are fostering innovation and accelerating the translation of research into commercial products. Furthermore, rising investments by venture capitalists and pharmaceutical companies in cell therapy companies are fueling market growth.
Opportunities
The cell therapy manufacturing market presents diverse opportunities for stakeholders. Expansion of manufacturing capacity to meet growing demand, particularly for personalized cell therapies, is a major opportunity. Moreover, the development of advanced manufacturing technologies, such as closed-system bioreactors and 3D cell culture systems, opens new avenues for process optimization and cost reduction. Additionally, expanding applications of cell therapies beyond oncology to autoimmune diseases and tissue regeneration offer promising opportunities for market expansion.
Challenges
Despite significant growth prospects, the cell therapy manufacturing market faces several challenges. Scalability remains a critical hurdle, with complexities in scaling up cell production while maintaining product quality and consistency. Regulatory complexities associated with the approval and commercialization of cell therapies pose another challenge, requiring stringent adherence to manufacturing standards and quality control measures. Additionally, high manufacturing costs and limited reimbursement policies in certain regions may impede market growth. Addressing these challenges will be essential for realizing the full potential of cell therapy manufacturing.
Read Also: Brazil Industrial Absorbent Market Size, Growth, Report by 2033
Cell Therapy Manufacturing Market Recent Developments
- In March 2024, Cellars announced the completion of the first Cell Shuttle, an automated, cGMP-compliant cell therapy manufacturing platform to support the global demand for cell therapies while reducing the cost of manufacturing and process failure rates.
- In March 2023, Cell One Partners and the Center for Breakthrough Medicines (CBM) entered into a strategic collaboration aimed at accelerating the development and commercialization of cell and gene therapies. This collaboration brings together the expertise and resources of both organizations to drive innovation and advance the field of regenerative medicine.
- In September 2022, the Cell Therapy Manufacturing Center (CTMC) and Ori Bio collaborated to expedite the process development, commercialization, and clinical integration of cell therapies. This partnership aimed to enhance the advancement of cell-based treatments and bring them to patients more efficiently.
Cell Therapy Manufacturing Market Companies
- Merck KGaA
- Thermo Fisher Scientific
- Catalent, Inc
- Bio-Techne
- Cytiva
- Lonza
- The Discovery Labs
- Novartis AG
- Bristol-Myers Squibb Company
- Gilead Sciences, Inc.
Segments Covered in the Report
By Therapy Type
- Allogenic Cell Therapy
- Autologous Cell Therapy
By Technology Type
- Somatic Cell Technology
- Cell Immortalization Technology
- Viral Vector Technology
- Genome Editing Technology
- Cell Plasticity Technology
- 3D Technology
By Source
- IPSC (Induced Pluripotent Stem Cell)
- Bone Marrow
- Umbilical Cord
- Adipose Tissues
- Neural Stem
By Application
- Musculoskeletal
- Cardiovascular
- Gastrointestinal
- Neurological
- Oncology
- Dermatology
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/