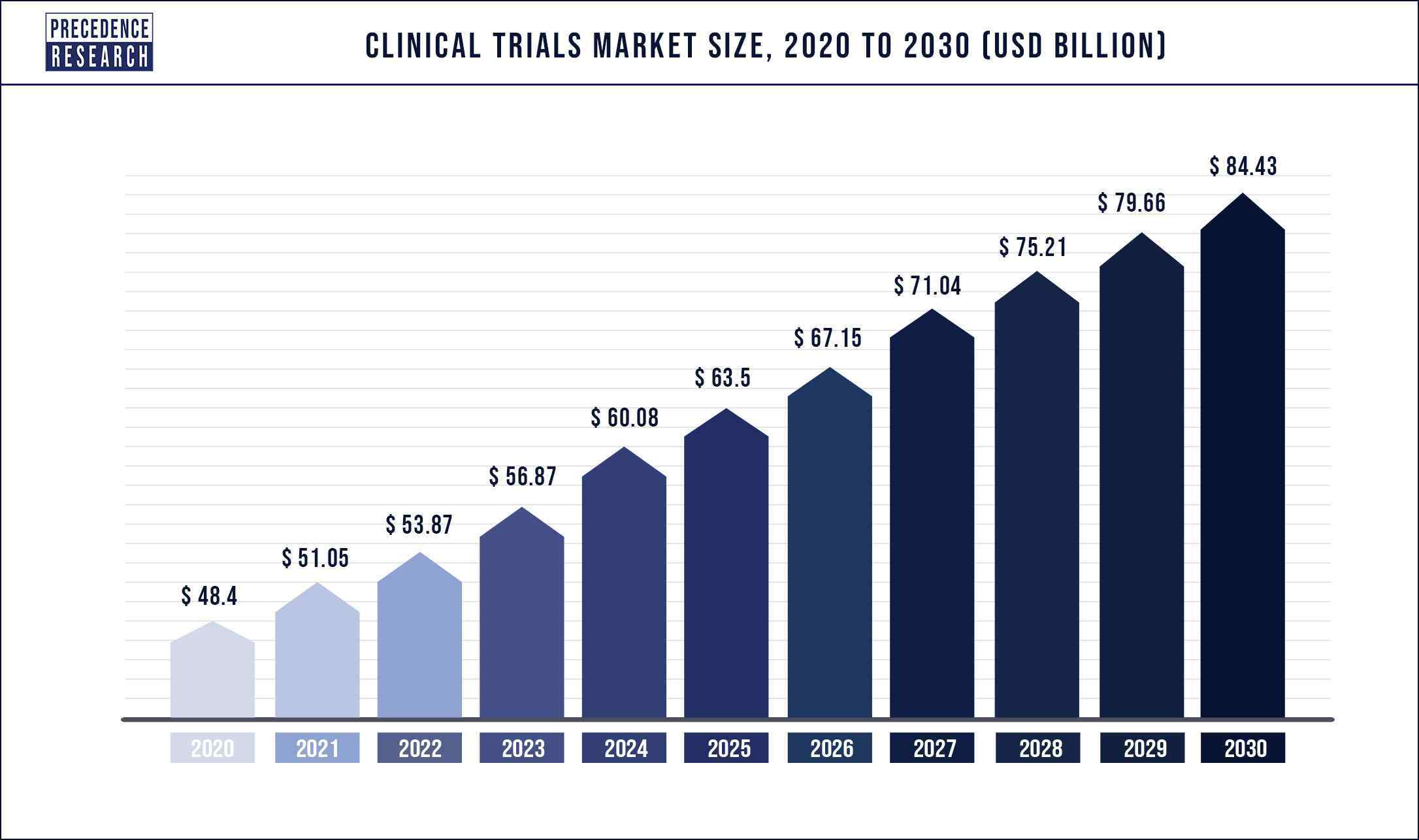
The clinical trials market size is predicted to reach USD 84.43 billion by 2030 and is expected to register a CAGR of 5.7% from 2022 to 2030.
The study provides an analysis of the period 2017-2030, wherein 2022 to 2030 is the forecast period and 2021 is considered as the base year.
The market revenue/volume with the help of widespread quantitative and qualitative insights, and forecasts of the market. This report presents a breakdown of the market into forthcoming and niche segments. Additionally, this research study gauges market revenue growth and its drift in global, regional, and country from 2017 to 2030.
The clinical trials market is expanding because to the rising frequency of chronic disorders and the rising demand for clinical trials in developing nations. The clinical trials market is fueled by the increasing number of biologics on the market and the demand for contract research organizations to undertake clinical studies. In addition, various biotechnology and pharmaceutical companies are now conducting clinical trials for severe chronic and infectious disorders such as HIV and cancer, which will help to expand the clinical trials market.
Download a Free Sample Copy of this Report@ https://www.precedenceresearch.com/sample/1185
Table of Contents
Report Scope of the Clinical Trials Market
| Report Highlights | Details |
| Market Size | USD 84.43 Billion by 2030 |
| Growth Rate | CAGR of 5.7% from 2021 to 2030 |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2021 |
| Forecast Period | 2021 to 2030 |
| Segments Covered | Phase, Study Design, Indication |
Market Dynamics
Drivers
Surge in demand for outsourcing clinical trials
The demand for efficient, fast-paced, and trustworthy clinical trials programs is expected to expand as the demand for novel medications and improved medical technologies grows. Furthermore, the medication development process is exceedingly dangerous for biotechnology and pharmaceutical businesses, with much lower approval rates and accompanying expansive expenses.
As a result, outsourcing the clinical trials program to several contract research organizations (CRO) has been determined to save the pharmaceutical corporation substantial time and money. Regional penetration of specific contract research organizations (CRO) has also been documented. Thus, the surge in the demand for outsourcing clinical trials is propelling the growth of the clinical trials market growth during the forecast period.
Restraints
High cost of clinical trials
The clinical trials market’s services are costly. In the forecast period, market labor costs are a restricting factor for the market growth. Patenting and contracting for the clinical trials market is a complicated process. As a result, the clinical trials market’s labor costs are high. The cost is an issue since it reduces demand in a few markets. The clinical trials services must be cost-effective in most businesses. The high cost, on the other hand, raises the industry’s overall operating costs. Thus, the high cost of clinical trials is hindering the growth of the clinical trials market during the forecast period.
Opportunities
Growing use of predictive analytics
Several firms are already using predictive analytics techniques such as artificial intelligence and machine learning to construct models and advise choices. Given the wealth of health data now available to clinical trial investigators, predictive analytics tools can be used in clinical trial design to identify patient characteristics that are more likely to respond to a specific treatment pattern, thereby increasing success rates and lowering risk in large, multi-center clinical trials. As a result, the growing use of predictive analysis is creating lucrative opportunities for the market growth during the forecast period.
Challenges
Stringent government regulations
Conducting clinical trials in different nations comes with a slew of regulatory challenges that could stymie market growth. The restrictions can be simplified with the cooperation of multiple regulatory authorities, however there is now a slow operating speed. Several nations also demand local language translation, as well as import and export authorization and the presentation of data on local patients. As a result, a complicated regulatory structure complicated regulatory structure combined with a considerable language barrier could stifle regional development.
Read Also: Wind Energy Market Size to Gain US$ 174.75 Billion by 2030
Report Highlights
- Based on the phase, the phase III segment dominated the global clinical trials market in 2020 with highest market share. The fact that a phase III study covers a high number of individuals and is the most expensive phase of a trial among all segments contributes to the clinical trials market growth.
- Based on the study design, the interventional design segment dominated the global clinical trials market in 2020 with highest market share. Interventional studies account largest percentage of all investigations, with the bulk of them using medicines or biologics, followed by clinical procedure, behavioral, and device intervention studies.
- Based on the indication, the oncology segment dominated the global clinical trials market in 2020 with highest market share. The oncology is the study of tumors. Tumors have the potential to be life-threatening in a large number of individuals, necessitating the development of better and more advanced treatments for diverse forms of tumors.
Regional Snapshot
Asia-Pacific is the largest segment for clinical trials market in terms of region. This is attributed to the growing availability of a broad patient pool, making candidate recruiting easier. The global COVID-19 pandemic is also a major contributor to the clinical trials market expansion.
North America region is the fastest growing region in the clinical trials market. This can be linked to the North America region’s increased research and development adoption of new clinical research technology. Furthermore, favorable government assistance for clinical trials in the U.S. is expected to stimulate demand.
Top Players contending in the Market:
The companies focusing on research and development are expected to lead the global clinical trials market. Leading competitors contending in global clinical trials market are as follows:
- Parexel
- IQVIA
- Charles River Laboratory
- Omnicare
- Kendle
- Chiltern
- Pharmaceutical Product Development, LLC
In order to better recognize the current status of clinical trials, and policies adopted by the foremost countries, Precedence Research predicted the future evolution of the clinical trials market. This research study bids qualitative and quantitative insights on clinical trials market and assessment of market size and growth trend for potential market segments.
Major Market Segments Covered:
By Phase
- Phase 1
- Phase 2
- Phase 3
- Phase 4
By Study Design
- Observational
- Interventional
- Expanded Access
By Indication
- Oncology
- Autoimmune/Inflammation
- Diabetes
- Central Nervous System
- Cardiovascular
- Pain Management
- Others
By Geography
-
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- United Kingdom
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of Middle East & Africa
- North America
Key Points Covered in clinical trials market Study:
- Growth of clinical trials in 2022
- Market Estimates and Forecasts (2017-2030)
- Market Share Analysis
- Key Drivers and Restraints Shaping Market Growth
- Segment-wise, Country-wise, and Region-wise Analysis
- Competition Mapping and Benchmarking
- Recommendation on Key Winning Strategies
- COVID-19 Impact on Demand for clinical trials and How to Navigate
- Key Product Innovations and Regulatory Climate
- Consumption Analysis
- Production Analysis
- Market and Management
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions& Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Clinical Trials Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
Chapter 5. COVID 19 Impact on Clinical Trials Market
5.1. Covid-19: Clinical Trials Industry Impact
5.2. Clinical Trials Business Impact Assessment: Covid-19
5.2.1. Services Challenges/Disruption
5.2.2. Market Trends and Clinical Trials Opportunities in the COVID-19 Landscape for Major Markets
5.3. Strategic Measures against Covid-19
5.3.1. Government Support and Initiative to Combat Covid-19
5.3.2. Proposal for Clinical Trials Market Players to deal with Covid-19 Pandemic Scenario
Chapter 6. Clinical Trials Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.1.1. Growing prevalence of chronic disorders
6.1.1.2. Increasing number of clinical trials in developing regions
6.1.2. Market Restraints
6.1.2.1. Rigorous guidelines
6.1.3. Market Opportunities
6.1.3.1. Increasing demand for advanced treatments such as personalized medicines
6.1.3.2. Technological advancements
Chapter 7. Global Clinical Trials Market: Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.1.1. Clinical Trials Market Revenue by Market Players (2016 -2019)
7.1.1.2. Clinical Trials Market Revenue Market Share by Market Players (2016 -2019)
7.1.2. Key Organic/Inorganic Strategies Adopted by Players
7.1.2.1. Phase Portfolio Expansion, Geographic Expansion, Phase Innovation
7.1.2.2. Merger and Acquisition, Collaboration and Partnerships
7.1.3. Market Players Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of End-users
Chapter 8. Global Clinical Trials Market, By Phase
8.1. Clinical Trials Market, by Phase, 2019 – 2030
8.1.1. Phase 1
8.1.1.1. Market Revenue and Forecast (2019 – 2030)
8.1.2. Phase 2
8.1.2.1. Market Revenue and Forecast (2019 – 2030)
8.1.3. Phase 3
8.1.3.1. Market Revenue and Forecast (2019 – 2030)
8.1.4. Phase 4
8.1.4.1. Market Revenue and Forecast (2019 – 2030)
Chapter 9. Global Clinical Trials Market, By Study Design
9.1. Clinical Trials Market, by Study Design, 2019 – 2030
9.1.1. Observational
9.1.1.1. Market Revenue and Forecast (2019 – 2030)
9.1.2. Interventional
9.1.2.1. Market Revenue and Forecast (2019 – 2030)
9.1.3. Expanded Access
9.1.3.1. Market Revenue and Forecast (2019 – 2030)
Chapter 10. Global Clinical Trials Market, By Indication
10.1. Clinical Trials Market, by Indication, 2019 – 2030
10.1.1. Oncology
10.1.1.1. Market Revenue and Forecast (2019 – 2030)
10.1.2. Autoimmune/Inflammation
10.1.2.1. Market Revenue and Forecast (2019 – 2030)
10.1.3. Diabetes
10.1.3.1. Market Revenue and Forecast (2019 – 2030)
10.1.4. Central Nervous System
10.1.4.1. Market Revenue and Forecast (2019 – 2030)
10.1.5. Cardiovascular
10.1.5.1. Market Revenue and Forecast (2019 – 2030)
10.1.6. Pain Management
10.1.7. Market Revenue and Forecast (2019 – 2030)
10.1.8. Others
10.1.8.1. Market Revenue and Forecast (2019 – 2030)
Chapter 11. Global Clinical Trials Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue Forecast by Phase(2019 – 2030)
11.1.2. Market Revenue Forecast by Study Design(2019 – 2030)
11.1.3. Market Revenue Forecast by Indication (2019 – 2030)
11.1.4. U.S
11.1.4.1. Market Revenue Forecast (2019 – 2030)
11.1.5. Canada
11.1.5.1. Market Revenue Forecast (2019 – 2030)
11.2. Europe
11.2.1. Market Revenue Forecast by Phase (2019 – 2030)
11.2.2. Market Revenue Forecast by Study Design (2019 – 2030)
11.2.3. Market Revenue Forecast by Indication (2019 – 2030)
11.2.4. UK
11.2.4.1. Market Revenue Forecast (2019 – 2030)
11.2.5. Germany
11.2.5.1. Market Revenue Forecast (2019 – 2030)
11.2.6. France
11.2.6.1. Market Revenue Forecast (2019 – 2030)
11.2.7. Rest of EU
11.2.7.1. Market Revenue Forecast (2019 – 2030)
11.3. Asia Pacific (APAC)
11.3.1. Market Revenue Forecast by Phase (2019 – 2030)
11.3.2. Market Revenue Forecast by Study Design (2019 – 2030)
11.3.3. Market Revenue Forecast by Indication (2019 – 2030)
11.3.4. China
11.3.4.1. Market Revenue Forecast (2019 – 2030)
11.3.5. India
11.3.5.1. Market Revenue Forecast (2019 – 2030)
11.3.6. Japan
11.3.6.1. Market Revenue Forecast (2019 – 2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue Forecast (2019 – 2030)
11.4. LATAM
11.4.1. Market Revenue Forecast by Phase (2019 – 2030)
11.4.2. Market Revenue Forecast by Study Design (2019 – 2030)
11.4.3. Market Revenue Forecast by Indication (2019 – 2030)
11.4.4. Brazil
11.4.4.1. Market Revenue Forecast (2019 – 2030)
11.4.5. Rest of LATAM
11.4.5.1. Market Revenue Forecast (2019 – 2030)
11.5. Middle East and Africa (MEA)
11.5.1. Market Revenue Forecast by Phase (2019 – 2030)
11.5.2. Market Revenue Forecast by Study Design (2019 – 2030)
11.5.3. Market Revenue Forecast by Indication (2019 – 2030)
11.5.4. GCC
11.5.4.1. Market Revenue Forecast (2019 – 2030)
11.5.5. North Africa
11.5.5.1. Market Revenue Forecast (2019 – 2030)
11.5.6. South Africa
11.5.6.1. Market Revenue Forecast (2019 – 2030)
11.5.7. Rest of MEA
11.5.7.1. Market Revenue Forecast (2019 – 2030)
Chapter 12. Company Profiles
12.1. Parexel
12.1.1. Company Overview, Business Information, Regional Presence
12.1.2. Phase Portfolio Analysis
12.1.2.1. Product Details, Specification, Indication
12.1.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.1.4. Recent Developments and Strategies
12.2. IQVIA
12.2.1. Company Overview, Business Information, Regional Presence
12.2.2. Phase Portfolio Analysis
12.2.2.1. Product Details, Specification, Indication
12.2.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.2.4. Recent Developments and Strategies
12.3. Charles River Laboratory
12.3.1. Company Overview, Business Information, Regional Presence
12.3.2. Phase Portfolio Analysis
12.3.2.1. Product Details, Specification, Indication
12.3.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.3.4. Recent Developments and Strategies
12.4. Omnicare
12.4.1. Company Overview, Business Information, Regional Presence
12.4.2. Phase Portfolio Analysis
12.4.2.1. Product Details, Specification, Indication
12.4.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.4.4. Recent Developments and Strategies
12.5. Kendle
12.5.1. Company Overview, Business Information, Regional Presence
12.5.2. Phase Portfolio Analysis
12.5.2.1. Product Details, Specification, Indication
12.5.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.5.4. Recent Developments and Strategies
12.6. Chiltern
12.6.1. Company Overview, Business Information, Regional Presence
12.6.2. Phase Portfolio Analysis
12.6.2.1. Product Details, Specification, Indication
12.6.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.6.4. Recent Developments and Strategies
12.7. Pharmaceutical Product Development, LLC
12.7.1. Company Overview, Business Information, Regional Presence
12.7.2. Phase Portfolio Analysis
12.7.2.1. Product Details, Specification, Indication
12.7.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.7.4. Recent Developments and Strategies
12.8. Company 8
12.8.1. Company Overview, Business Information, Regional Presence
12.8.2. Phase Portfolio Analysis
12.8.2.1. Product Details, Specification, Indication
12.8.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.8.4. Recent Developments and Strategies
12.9. Company 9
12.9.1. Company Overview, Business Information, Regional Presence
12.9.2. Phase Portfolio Analysis
12.9.2.1. Product Details, Specification, Indication
12.9.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.9.4. Recent Developments and Strategies
12.10. Company 10
12.10.1. Company Overview, Business Information, Regional Presence
12.10.2. Phase Portfolio Analysis
12.10.2.1. Product Details, Specification, Indication
12.10.3. Revenue, Price, and Gross Margin (2019 – 2020)
12.10.4. Recent Developments and Strategies
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Buy Full Research Report (Single User License US$ 4500) @ https://www.precedenceresearch.com/checkout/1185
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Website: https://www.precedenceresearch.com
