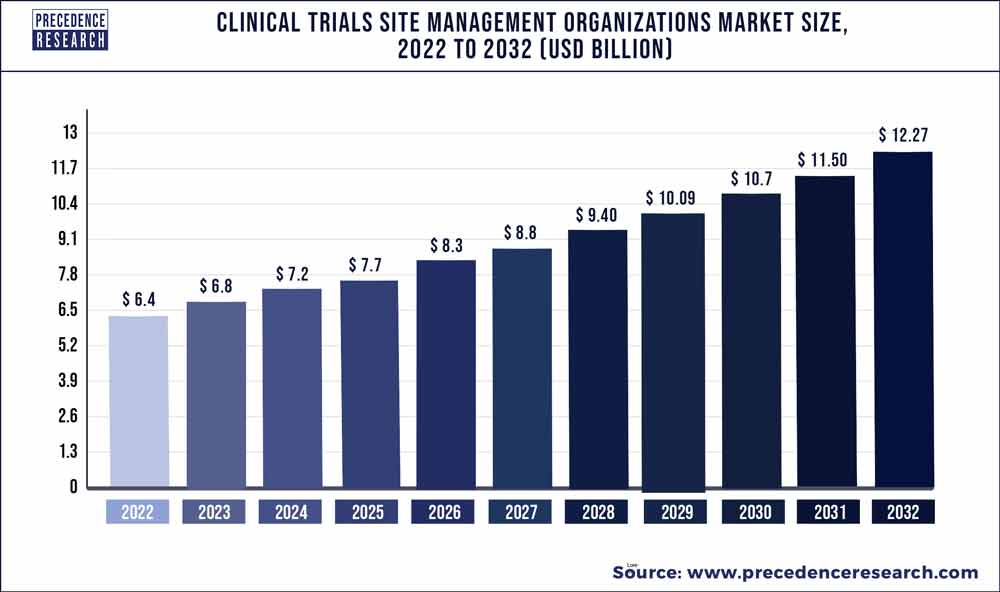
The global clinical trials site management organizations market size was approximate at US$ 6.4 billion in 2022 and is anticipated to grow US$ 6 billion by 2032, registering a compound annual growth rate of 5% from 2023 to 2032.
The clinical trials site management organizations market report covering various industry elements and growth trends helpful for predicting the market’s future.
The study provides a strong base for the clinical trials site management organizations market to be segmented into different segments. In fact, the study also covers the maximum market share during the assessment period by 2032.
This study is based on the partners that are highly competitive, key players as well as their market revenue in the forecast years of 2023 g0066ztr to 2032. There is also a strong focus on product revenues, sales, product categories and even the products that are experiencing the most traction. In this manner, the clinical trials site management organizations report also speaks about the effectiveness of this market along with its growth during the forecast period of 2030. Other major attributes of the clinical trials site management organizations market have been studied and analysed across many developments.
Request a Sample Copy of This Report @ https://www.precedenceresearch.com/sample/2960
Clinical Trials Site Management Organizations Market Report Scope
| Report Coverage | Details |
| Market Size in 2023 | USD 6.8 Billion |
| Market Size by 2032 | USD 12.27 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 6.72% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | By Clinical Trial Services, By Phase, and By Therapeutic Area |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Also read: Electron Microscopy Market Size to Grow US$ 14.06 Billion By 2032
Research Approach
The comprehensive report on the global clinical trials site management organizations market begins with an overview, followed by the scope and objectives of the study. The report provides detailed explanation of the objectives behind this study and key vendors and distributors operating in the market and regulatory scenario for approval of products. Following this, the report provides detailed explanation of objectives of this study and laid down by accredited agencies in the purview of research in the global clinical trials site management organizations market.
It is followed by market introduction, market dynamics, and an overview of the global market, which includes analysis of market drivers, restraints, and trends pertaining to the global market. Furthermore, Y-o-Y growth analysis with elaborated insights has been provided in order to understand the Y-o-Y growth trend of the global market.
For reading comprehensibility, the report has been compiled in a chapter-wise layout, with each section divided into smaller sections. The report comprises an exhaustive collection of graphs and tables that are appropriately interspersed. Pictorial representation of actual and projected values of key segments is visually appealing to readers. This also allows comparison of the market shares of key segments in the past and at the end of the forecast period.
Key Players
- Clinedge
- WCG
- ClinChoice
- Access Clinical Research
- FOMAT Medical Research INC.
- SGS
- KV Clinical
- SMO-Pharmina
- Xylem Clinical Research
- Aurum Clinical Research
Clinical Trials Site Management Organizations Market Segmentations
By Clinical Trial Services
- Site Management
- Project Management
- Regulatory
- Onsite monitoring
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Therapeutic Area
- Oncology
- Cardiology
- CNS
- Pain management
- Endocrine
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology (Premium Insights)
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Clinical Trials Site Management Organizations Market
5.1. COVID-19 Landscape: Clinical Trials Site Management Organizations Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Clinical Trials Site Management Organizations Market, By Clinical Trial Services
8.1. Clinical Trials Site Management Organizations Market, by Clinical Trial Services, 2023-2032
8.1.1 Site Management
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Project Management
8.1.2.1. Market Revenue and Forecast (2020-2032)
8.1.3. Regulatory
8.1.3.1. Market Revenue and Forecast (2020-2032)
8.1.4. Onsite monitoring
8.1.4.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global Clinical Trials Site Management Organizations Market, By Phase
9.1. Clinical Trials Site Management Organizations Market, by Phase, 2023-2032
9.1.1. Phase I
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Phase II
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Phase III
9.1.3.1. Market Revenue and Forecast (2020-2032)
9.1.4. Phase IV
9.1.4.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global Clinical Trials Site Management Organizations Market, By Therapeutic Area
10.1. Clinical Trials Site Management Organizations Market, by Therapeutic Area, 2023-2032
10.1.1. Oncology
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Cardiology
10.1.2.1. Market Revenue and Forecast (2020-2032)
10.1.3. CNS
10.1.3.1. Market Revenue and Forecast (2020-2032)
10.1.4. Pain management
10.1.4.1. Market Revenue and Forecast (2020-2032)
10.1.5. Endocrine
10.1.5.1. Market Revenue and Forecast (2020-2032)
Chapter 11. Global Clinical Trials Site Management Organizations Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.1.2. Market Revenue and Forecast, by Phase (2020-2032)
11.1.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.1.4.2. Market Revenue and Forecast, by Phase (2020-2032)
11.1.4.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.1.5.2. Market Revenue and Forecast, by Phase (2020-2032)
11.1.5.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.2.2. Market Revenue and Forecast, by Phase (2020-2032)
11.2.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.2.4.2. Market Revenue and Forecast, by Phase (2020-2032)
11.2.4.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.2.5.2. Market Revenue and Forecast, by Phase (2020-2032)
11.2.5.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.2.6.2. Market Revenue and Forecast, by Phase (2020-2032)
11.2.6.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.2.7.2. Market Revenue and Forecast, by Phase (2020-2032)
11.2.7.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.3.2. Market Revenue and Forecast, by Phase (2020-2032)
11.3.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.3.4.2. Market Revenue and Forecast, by Phase (2020-2032)
11.3.4.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.3.5.2. Market Revenue and Forecast, by Phase (2020-2032)
11.3.5.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.3.6.2. Market Revenue and Forecast, by Phase (2020-2032)
11.3.6.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.3.7.2. Market Revenue and Forecast, by Phase (2020-2032)
11.3.7.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.4.2. Market Revenue and Forecast, by Phase (2020-2032)
11.4.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.4.4.2. Market Revenue and Forecast, by Phase (2020-2032)
11.4.4.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.4.5.2. Market Revenue and Forecast, by Phase (2020-2032)
11.4.5.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.4.6.2. Market Revenue and Forecast, by Phase (2020-2032)
11.4.6.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.4.7.2. Market Revenue and Forecast, by Phase (2020-2032)
11.4.7.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.5.2. Market Revenue and Forecast, by Phase (2020-2032)
11.5.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.5.4.2. Market Revenue and Forecast, by Phase (2020-2032)
11.5.4.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Clinical Trial Services (2020-2032)
11.5.5.2. Market Revenue and Forecast, by Phase (2020-2032)
11.5.5.3. Market Revenue and Forecast, by Therapeutic Area (2020-2032)
Chapter 12. Company Profiles
12.1. Clinedge
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. WCG
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. ClinChoice
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Access Clinical Research
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. FOMAT Medical Research INC.
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. SGS
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. KV Clinical
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. SMO-Pharmina
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Xylem Clinical Research
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Aurum Clinical Research
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Why should you invest in this report?
If you are aiming to enter the global clinical trials site management organizations market, this report is a comprehensive guide that provides crystal clear insights into this niche market. All the major application areas for clinical trials site management organizations are covered in this report and information is given on the important regions of the world where this market is likely to boom during the forecast period of 2023-2030 so that you can plan your strategies to enter this market accordingly.
Besides, through this report, you can have a complete grasp of the level of competition you will be facing in this hugely competitive market and if you are an established player in this market already, this report will help you gauge the strategies that your competitors have adopted to stay as market leaders in this market. For new entrants to this market, the voluminous data provided in this report is invaluable.
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
