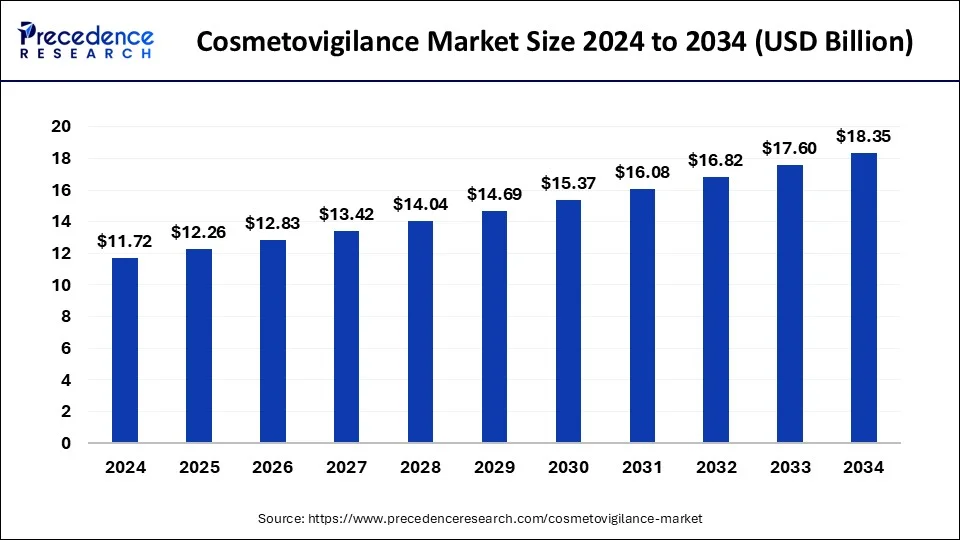
The global cosmetovigilance market size was estimated at USD 11.20 billion in 2023 and is projected to attain around USD 17.60 billion by 2033, growing at a CAGR of 4.62% from 2024 to 2033.
Key Points
- Europe has captured highest revenue share in cosmetovigilance market in 2023 with 37.8%.
- By service type, the post-marketing services market segment accounted for the dominant share of the market in 2023 with 64%.
- By service type, the pre-marketing services segment is expected to witness considerable growth in the global market over the forecast period.
- By categories, the skincare segment held the largest share of around 33% in 2023.
- By service provider, the contract outsourcing segment has accounted largest revenue share of 60% in 2023.
- By phase type, the Phase IV segment has held revenue share of 76% in 2023.
The Cosmetovigilance Market encompasses the surveillance, assessment, and monitoring of cosmetic products to ensure consumer safety and regulatory compliance. With the increasing demand for cosmetic products worldwide, driven by factors such as rising disposable income, changing consumer preferences, and growing awareness of personal grooming, the need for robust cosmetovigilance measures has become paramount. This overview delves into the trends, growth factors, opportunities, challenges, and region-specific insights shaping the cosmetovigilance market.
Get a Sample: https://www.precedenceresearch.com/sample/4062
Growth Factors:
The growth of the cosmetovigilance market is propelled by several factors. Firstly, stringent regulations and safety standards imposed by regulatory bodies, such as the FDA (Food and Drug Administration) in the United States and the European Commission in Europe, mandate the surveillance and reporting of adverse effects associated with cosmetic products. Compliance with these regulations necessitates the implementation of robust cosmetovigilance systems by cosmetic manufacturers, driving market growth.
Moreover, increasing consumer awareness regarding product ingredients, potential allergens, and adverse reactions has fueled demand for safer and more transparent cosmetic products. Manufacturers are increasingly investing in research and development to develop innovative, high-quality products while adhering to safety standards, thereby driving the adoption of cosmetovigilance practices.
Region Insights:
The cosmetovigilance market exhibits region-specific dynamics influenced by factors such as regulatory frameworks, consumer preferences, and market maturity. In North America, stringent regulatory oversight by agencies such as the FDA and growing consumer awareness of product safety drive the adoption of cosmetovigilance practices among cosmetic manufacturers. The presence of key market players and advanced healthcare infrastructure further bolsters market growth in the region.
In Europe, the European Union’s comprehensive regulatory framework, including the Cosmetics Regulation (EC) No 1223/2009, mandates stringent safety assessments and reporting requirements for cosmetic products. As a result, European cosmetic manufacturers prioritize cosmetovigilance to ensure compliance and maintain consumer trust. Additionally, growing consumer demand for natural, organic, and ethically sourced cosmetic products underscores the importance of transparency and safety in the European market.
In the Asia-Pacific region, rapid urbanization, rising disposable incomes, and changing consumer lifestyles drive the demand for cosmetic products. However, varying regulatory frameworks across countries and a fragmented market landscape pose challenges for cosmetovigilance implementation. Nevertheless, the increasing focus on product quality, safety, and regulatory compliance presents opportunities for cosmetovigilance service providers to expand their presence in the region.
Cosmetovigilance Market Scope
| Report Coverage | Details |
| Global Market Size in 2023 | USD 11.20 Billion |
| Global Market Size in 2024 | USD 11.72 Billion |
| Global Market Size by 2033 | USD 17.60 Billion |
| Growth Rate from 2024 to 2033 | CAGR of 4.62% |
| Largest Market | Europe |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Service Type, By Categories, By Phase Type, and By Service Provider |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Cosmetovigilance Market Dynamics
Drivers:
Several drivers propel the growth of the cosmetovigilance market. Firstly, the increasing prevalence of adverse reactions and allergic responses to cosmetic products necessitates stringent surveillance and monitoring to ensure consumer safety. Reports of adverse events, such as skin irritation, allergic dermatitis, or even more severe reactions, underscore the importance of proactive cosmetovigilance measures.
Furthermore, the growing trend towards personalized skincare and beauty products, driven by advancements in technology and consumer preferences, creates opportunities and challenges for cosmetovigilance. Customized formulations, active ingredients, and novel delivery systems require thorough safety assessments and monitoring to mitigate potential risks and ensure product efficacy.
Opportunities:
Despite the challenges, the cosmetovigilance market presents significant opportunities for stakeholders. Firstly, advancements in technology, such as artificial intelligence, machine learning, and big data analytics, enhance the efficiency and accuracy of adverse event detection and monitoring. Automated surveillance systems can analyze vast amounts of data from various sources, including social media, consumer feedback, and regulatory databases, to identify potential safety concerns promptly.
Moreover, the growing trend towards green and sustainable cosmetics creates opportunities for cosmetovigilance service providers to develop eco-friendly, cruelty-free, and ethically sourced products. Collaborations with regulatory agencies, consumer advocacy groups, and industry associations can further enhance transparency, compliance, and consumer trust in cosmetic products.
Challenges:
Despite the opportunities, the cosmetovigilance market faces several challenges. Firstly, the global nature of the cosmetic industry, coupled with varying regulatory frameworks across countries, poses challenges for harmonization and standardization of cosmetovigilance practices. Divergent reporting requirements, terminology, and data collection methodologies hinder seamless information sharing and collaboration among stakeholders.
Moreover, the rapid proliferation of cosmetic products, fueled by e-commerce platforms and direct-to-consumer brands, increases the complexity and volume of data to be monitored and analyzed. Identifying and prioritizing potential safety signals amidst the vast array of products and ingredients requires advanced analytical tools and expertise, which may pose challenges for smaller manufacturers and regulatory agencies with limited resources.
Read Also: IT Services Market Size to Rake USD 2.80 Trillion By 2033
Recent Developments
- In December 2023, Biofrontera AG, an international biopharmaceutical company, announced that its wholly-owned subsidiary Biofrontera Bioscience GmbH has received a notice of allowance for the application “Photodynamic therapy comprising two light exposures at different wavelengths by the US patent office USPTO. This patent protects several innovations relating to a new illumination method to treat dermatological skin diseases with Photodynamic Therapy (PDT).
- In June 2023, Anju Software and ClinChoice partnered to enhance eClinical solutions for clinical research. ClinChoice’s acquisition of Cromsource led to the collaboration, enabling the delivery of adaptable and cost-efficient eClinical solutions, including TrialMaster, for streamlined clinical trial data management and regulatory compliance.
Cosmetovigilance Market Companies
- Poseidon CRO
- AxeRegel
- PharSafer
- AB Cube
- Aixial Group
- Di Renzo
- Pharmathen
- Skill Pharma
- OC Vigilance
- Preclinical
- safety
- ZEINCRO Group
- FMD K&L
- POSEIDON CRO
- MSL Solution Providers
- Cliantha
- Pure Drug Safety
- KMJ pharma sp. z o.o.
- TheraSkin
- CORONIS Research SA
- PHARMALEX GMBH
- Bioclinica
- Tecnimede Group
- Sciformix
Segments Covered in the Report
By Service Type
- Pre-marketing Services
- Clinical Safety Testing
- Document Writing
- Risk Management
- Post-marketing Services
- Case Intake
- Case Triage
- Data Entry & Acquisition
- Tracking & Reporting
By Categories
- Skincare
- Makeup
- Haircare
- Perfumes and deodorants
- Hair colorants
- Others
By Phase Type
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
By Service Provider
- In-house
- Contract Outsourcing
- Contract Research Organizations (CROs)
- Business Process Outsourcing Organizations (BPOs)
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/