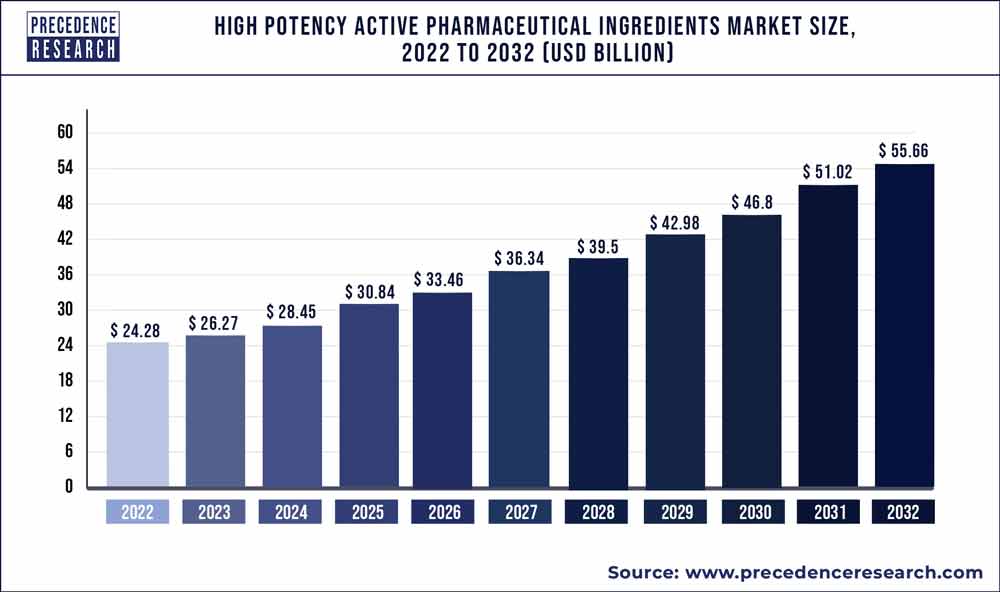
The global high potency active pharmaceutical ingredients market size was valued at US$ 23.6 billion in 2021 and is anticipated to grow US$ 53.8 billion by 2030, registering a compound annual growth rate of 9.59% from 2022 to 2030.
The high potency active pharmaceutical ingredients market report covering various industry elements and growth trends helpful for predicting the market’s future.
The study provides a strong base for the high potency active pharmaceutical ingredients market to be segmented into different segments. In fact, the study also covers the maximum market share during the assessment period by 2030.
This study is based on the partners that are highly competitive, key players as well as their market revenue in the forecast years of 2022 to 2030. There is also a strong focus on product revenues, sales, product categories and even the products that are experiencing the most traction. In this manner, the high potency active pharmaceutical ingredients report also speaks about the effectiveness of this market along with its growth during the forecast period of 2030. Other major attributes of the high potency active pharmaceutical ingredients market have been studied and analysed across many developments.
High Potency Active Pharmaceutical Ingredients Market Scope
| Report Coverage | Details |
| Market Size in 2022 | USD 25.86 Billion |
| Market Size by 2030 | USD 53.8 Billion |
| Growth Rate from 2022 to 2030 | CAGR of 9.59% |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered |
|
Also read: Nitric Acid Market Size to Grow US$ 39.7 Billion By 2030
Research Approach
The comprehensive report on the global high potency active pharmaceutical ingredients market begins with an overview, followed by the scope and objectives of the study. The report provides detailed explanation of the objectives behind this study and key vendors and distributors operating in the market and regulatory scenario for approval of products. Following this, the report provides detailed explanation of objectives of this study and laid down by accredited agencies in the purview of research in the global high potency active pharmaceutical ingredients market.
It is followed by market introduction, market dynamics, and an overview of the global market, which includes analysis of market drivers, restraints, and trends pertaining to the global market. Furthermore, Y-o-Y growth analysis with elaborated insights has been provided in order to understand the Y-o-Y growth trend of the global market.
For reading comprehensibility, the report has been compiled in a chapter-wise layout, with each section divided into smaller sections. The report comprises an exhaustive collection of graphs and tables that are appropriately interspersed. Pictorial representation of actual and projected values of key segments is visually appealing to readers. This also allows comparison of the market shares of key segments in the past and at the end of the forecast period.
Key Players
- BASF SE
- CordenPharma
- Dr. Reddy’s Laboratories Ltd.
- CARBOGEN AMCIS AG
- Pfizer, Inc.
- Sun Pharmaceutical Industries, Ltd.
- Teva Pharmaceutical Industries Ltd.
- Albany Molecular Research, Inc.
- Sanofi S.A.
- Merck & Co., Inc.
- Novartis AG
High Potency Active Pharmaceutical Ingredients Market Segmentations
By Product
- Synthetic
- Biotech
By Manufacturer Type
- In-house
- Outsourced
By Drug Type
- Innovative
- Generic
By Application
- Oncology
- Hormonal Disorders
- Glaucoma
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on High Potency Active Pharmaceutical Ingredients Market
5.1. COVID-19 Landscape: High Potency Active Pharmaceutical Ingredients Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global High Potency Active Pharmaceutical Ingredients Market, By Product
8.1. High Potency Active Pharmaceutical Ingredients Market, by Product, 2022-2030
8.1.1. Synthetic
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Biotech
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global High Potency Active Pharmaceutical Ingredients Market, By Manufacturer Type
9.1. High Potency Active Pharmaceutical Ingredients Market, by Manufacturer Type, 2022-2030
9.1.1. In-house
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Outsourced
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global High Potency Active Pharmaceutical Ingredients Market, By Drug Type
10.1. High Potency Active Pharmaceutical Ingredients Market, by Drug Type, 2022-2030
10.1.1. Innovative
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Generic
10.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global High Potency Active Pharmaceutical Ingredients Market, By Application
11.1. High Potency Active Pharmaceutical Ingredients Market, by Application, 2022-2030
11.1.1. Oncology
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Hormonal Disorders
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Glaucoma
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Others
11.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global High Potency Active Pharmaceutical Ingredients Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Product (2017-2030)
12.1.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.1.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.4. Market Revenue and Forecast, by Application (2017-2030)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.1.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.1.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.1.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.1.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.6.4. Market Revenue and Forecast, by Application (2017-2030)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.4. Market Revenue and Forecast, by Application (2017-2030)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.6.4. Market Revenue and Forecast, by Application (2017-2030)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.7.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.7.4. Market Revenue and Forecast, by Application (2017-2030)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Product (2017-2030)
12.2.8.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.2.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.8.4. Market Revenue and Forecast, by Application (2017-2030)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.4. Market Revenue and Forecast, by Application (2017-2030)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.6.4. Market Revenue and Forecast, by Application (2017-2030)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.7.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.7.4. Market Revenue and Forecast, by Application (2017-2030)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Product (2017-2030)
12.3.8.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.3.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.8.4. Market Revenue and Forecast, by Application (2017-2030)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.4. Market Revenue and Forecast, by Application (2017-2030)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.6.4. Market Revenue and Forecast, by Application (2017-2030)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.7.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.7.4. Market Revenue and Forecast, by Application (2017-2030)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Product (2017-2030)
12.4.8.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.4.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.8.4. Market Revenue and Forecast, by Application (2017-2030)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Product (2017-2030)
12.5.5.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.5.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.5.4. Market Revenue and Forecast, by Application (2017-2030)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Product (2017-2030)
12.5.6.2. Market Revenue and Forecast, by Manufacturer Type (2017-2030)
12.5.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.6.4. Market Revenue and Forecast, by Application (2017-2030)
Chapter 13. Company Profiles
13.1. BASF SE
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. CordenPharma
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Dr. Reddy’s Laboratories Ltd.
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. CARBOGEN AMCIS AG
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Pfizer, Inc.
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Sun Pharmaceutical Industries, Ltd.
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Teva Pharmaceutical Industries Ltd.
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Albany Molecular Research, Inc.
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Sanofi S.A.
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Merck & Co., Inc.
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
Why should you invest in this report?
If you are aiming to enter the global high potency active pharmaceutical ingredients market, this report is a comprehensive guide that provides crystal clear insights into this niche market. All the major application areas for high potency active pharmaceutical ingredients are covered in this report and information is given on the important regions of the world where this market is likely to boom during the forecast period of 2022-2030 so that you can plan your strategies to enter this market accordingly.
Besides, through this report, you can have a complete grasp of the level of competition you will be facing in this hugely competitive market and if you are an established player in this market already, this report will help you gauge the strategies that your competitors have adopted to stay as market leaders in this market. For new entrants to this market, the voluminous data provided in this report is invaluable.
Request a Sample Copy of This Report @https://www.precedenceresearch.com/sample/2324
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
