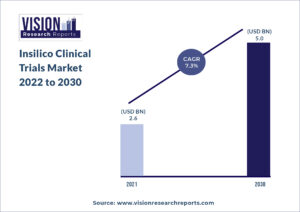
The global insilico clinical trials market size was valued at USD 2.6 billion in 2021, and is predicted to be worth around USD 5.0 billion by 2030, registering a CAGR of 7.3% during the forecast period 2022 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/39181
Table of Contents
Insilico Clinical Trials Market Growth Factors
Traditional clinical trials require huge expenditure to conduct research. Moreover, a high number of drugs and medical devices fail in the clinical trial owing to the lack of safety and efficacy, which creates huge losses for the clinical trial sponsors. These factors promote the demand for insilico clinical trials, to understand the behavior of drugs or medical devices in humans. Insilico clinical trials use simulation techniques to understand the efficacy and safety of a drug or medical device.
The growth in R&D spending is likely to have a positive impact on the market growth. The COVID-19 pandemic had resulted in a temporary shutdown of clinical research sites, which promoted the demand for insilico clinical trials for research studies. Disruptions in clinical research due to the COVID-19 outbreak have kindled a new level of interest in using computer simulations to predict clinical trial outcomes.
Report Coverage
| Report Scope | Details |
| Market Size | US$ 5.0 billion by 2030 |
| Growth Rate | CAGR of 7.3% From 2022 to 2030 |
| Largest Market | North America |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Industry, Therapeutic Area, Phase |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | Caterpillar; CNH Industrial N.V.; Doosan Corporation; Escorts Limited; Hitachi Construction Machinery Co., Ltd.; Hyundai Construction Equipment Co., Ltd.; J C Bamford Excavators Ltd.; Deere & Company.; Kobelco Construction Machinery Co., Ltd.; Komatsu Ltd.; Liebherr-International AG; Manitou BF; HİDROMEK; Sany Heavy Industry Co., Ltd.; Sumitomo Heavy Industries, Ltd.; Terex Corporation; Volvo AB; Zoomlion Heavy Industry Science; Technology Co., Ltd |
By Industry Analysis
The medical devices segment accounted for the highest revenue share of more than 57% in 2021. The simulations for medical devices are considered more accurate as compared to pharmaceuticals, which is the key reason for the majority of insilico trials being performed for medical devices.
pharmaceutical segment is expected to grow at the fastest CAGR over the forecast period owing to the increasing demand for innovative treatment options globally. The concerns regarding the harmful effect of drugs on humans in traditional clinical trials are further contributing to the demand for computer simulation trials for pharmaceuticals.
By Therapeutic Area Analysis
Based on therapeutic areas, the market has been further segmented into oncology, infectious disease, hematology, cardiology, dermatology, neurology, diabetes, and others. The oncology segment accounted for the largest revenue share of 27.0% in 2021.
Traditional clinical trials for cancer are considered expensive and also have high chances of incurring harmful effects on humans. These factors are primarily contributing to the demand for cancer insilico clinical trials
The developments in cancer insilico studies have further promoted segment growth. For instance, in May 2021, GNS Healthcare announced the development of the first in silico patient for prostate cancer. However, the infectious diseases segment is expected to register the fastest CAGR over the forecast period.
By Phase Analysis
Based on phases, the market is divided into phases I, II, III, and IV. The phase II segment accounted for the largest revenue share of more than 44.5% in 2021 and is also expected to register the fastest CAGR during the forecast period. The majority of insilico trials are at phase II, which is the prime reason for the largest share of the segment.
The phase II segment is also expected to record significant growth during the forecast period. The rising demand for biologics and personalized medicines across the globe and increasing awareness among the population to eliminate animal studies are a few of the factors supporting the growth of the phase III segment.
By Regional Analysis
North America accounted for the largest revenue share of more than 44% in 2021 and will expand further at a steady CAGR retaining the leading over the forecast period as a significant number of insilico trials are being conducted in the U.S. In addition, the presence of key companies like GNS Healthcare Inc., Insilico Medicine, Inc., Immunetrics Inc., and others in the region have further contributed to the regional market growth.
The market in Asia Pacific is in its nascent stage. Various companies in this region are adopting the use of insilico trials to reduce the overall cost of clinical studies.
Read also @ Microdermabrasion Devices Market Impressive Gains by 2022-2028
Major Key Players Covered in The Insilico Clinical Trials Market Report include
- Certara, Inc.
- Novadiscovery Sas
- Insilico Medicine, Inc.
- Dassault Systemes SE
- GNS Healthcare Inc.
- The AnyLogic Company
- InSilicoTrials
- Immunetrics Inc.
- Nuventra Pharma Sciences
- Abzena Ltd.
Insilico Clinical Trials Market Segmentation
- By Industry
- Medical Devices
- Pharmaceutical
- By Therapeutic Area
- Oncology
- Infectious Disease
- Hematology
- Cardiology
- Dermatology
- Neurology
- Diabetes
- Others
- By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
- Regional
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Spain
- Italy
- Asia Pacific
- India
- China
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Insilico Clinical Trials Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Insilico Clinical Trials Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Insilico Clinical Trials Market, By Industry
7.1. Insilico Clinical Trials Market, by Industry, 2021-2030
7.1.1. Medical Devices
7.1.1.1. Market Revenue and Forecast (2019-2030)
7.1.2. Pharmaceutical
7.1.2.1. Market Revenue and Forecast (2019-2030)
Chapter 8. Global Insilico Clinical Trials Market, By Therapeutic Area
8.1. Insilico Clinical Trials Market, by Therapeutic Area, 2021-2030
8.1.1. Oncology
8.1.1.1. Market Revenue and Forecast (2019-2030)
8.1.2. Infectious Disease
8.1.2.1. Market Revenue and Forecast (2019-2030)
8.1.3. Hematology
8.1.3.1. Market Revenue and Forecast (2019-2030)
8.1.4. Cardiology
8.1.4.1. Market Revenue and Forecast (2019-2030)
8.1.5. Dermatology
8.1.5.1. Market Revenue and Forecast (2019-2030)
8.1.6. Neurology
8.1.6.1. Market Revenue and Forecast (2019-2030)
8.1.7. Diabetes
8.1.7.1. Market Revenue and Forecast (2019-2030)
Chapter 9. Global Insilico Clinical Trials Market, By Phase
9.1. Insilico Clinical Trials Market, by Phase, 2021-2030
9.1.1. Phase I
9.1.1.1. Market Revenue and Forecast (2019-2030)
9.1.2. Phase II
9.1.2.1. Market Revenue and Forecast (2019-2030)
9.1.3. Phase III
9.1.3.1. Market Revenue and Forecast (2019-2030)
9.1.4. Phase IV
9.1.4.1. Market Revenue and Forecast (2019-2030)
Chapter 10. Global Insilico Clinical Trials Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Industry (2019-2030)
10.1.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.1.3. Market Revenue and Forecast, by Phase (2019-2030)
10.1.4. U.S.
10.1.4.1. Market Revenue and Forecast, by Industry (2019-2030)
10.1.4.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.1.4.3. Market Revenue and Forecast, by Phase (2019-2030)
10.1.5. Rest of North America
10.1.5.1. Market Revenue and Forecast, by Industry (2019-2030)
10.1.5.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.1.5.3. Market Revenue and Forecast, by Phase (2019-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Industry (2019-2030)
10.2.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.2.3. Market Revenue and Forecast, by Phase (2019-2030)
10.2.4. UK
10.2.4.1. Market Revenue and Forecast, by Industry (2019-2030)
10.2.4.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.2.4.3. Market Revenue and Forecast, by Phase (2019-2030)
10.2.5. Germany
10.2.5.1. Market Revenue and Forecast, by Industry (2019-2030)
10.2.5.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.2.5.3. Market Revenue and Forecast, by Phase (2019-2030)
10.2.6. France
10.2.6.1. Market Revenue and Forecast, by Industry (2019-2030)
10.2.6.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.2.6.3. Market Revenue and Forecast, by Phase (2019-2030)
10.2.7. Rest of Europe
10.2.7.1. Market Revenue and Forecast, by Industry (2019-2030)
10.2.7.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.2.7.3. Market Revenue and Forecast, by Phase (2019-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Industry (2019-2030)
10.3.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.3.3. Market Revenue and Forecast, by Phase (2019-2030)
10.3.4. India
10.3.4.1. Market Revenue and Forecast, by Industry (2019-2030)
10.3.4.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.3.4.3. Market Revenue and Forecast, by Phase (2019-2030)
10.3.5. China
10.3.5.1. Market Revenue and Forecast, by Industry (2019-2030)
10.3.5.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.3.5.3. Market Revenue and Forecast, by Phase (2019-2030)
10.3.6. Japan
10.3.6.1. Market Revenue and Forecast, by Industry (2019-2030)
10.3.6.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.3.6.3. Market Revenue and Forecast, by Phase (2019-2030)
10.3.7. Rest of APAC
10.3.7.1. Market Revenue and Forecast, by Industry (2019-2030)
10.3.7.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.3.7.3. Market Revenue and Forecast, by Phase (2019-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Industry (2019-2030)
10.4.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.4.3. Market Revenue and Forecast, by Phase (2019-2030)
10.4.4. GCC
10.4.4.1. Market Revenue and Forecast, by Industry (2019-2030)
10.4.4.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.4.4.3. Market Revenue and Forecast, by Phase (2019-2030)
10.4.5. North Africa
10.4.5.1. Market Revenue and Forecast, by Industry (2019-2030)
10.4.5.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.4.5.3. Market Revenue and Forecast, by Phase (2019-2030)
10.4.6. South Africa
10.4.6.1. Market Revenue and Forecast, by Industry (2019-2030)
10.4.6.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.4.6.3. Market Revenue and Forecast, by Phase (2019-2030)
10.4.7. Rest of MEA
10.4.7.1. Market Revenue and Forecast, by Industry (2019-2030)
10.4.7.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.4.7.3. Market Revenue and Forecast, by Phase (2019-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Industry (2019-2030)
10.5.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.5.3. Market Revenue and Forecast, by Phase (2019-2030)
10.5.4. Brazil
10.5.4.1. Market Revenue and Forecast, by Industry (2019-2030)
10.5.4.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.5.4.3. Market Revenue and Forecast, by Phase (2019-2030)
10.5.5. Rest of LATAM
10.5.5.1. Market Revenue and Forecast, by Industry (2019-2030)
10.5.5.2. Market Revenue and Forecast, by Therapeutic Area (2019-2030)
10.5.5.3. Market Revenue and Forecast, by Phase (2019-2030)
Chapter 11. Company Profiles
11.1. Certara, Inc.
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Novadiscovery Sas
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Insilico Medicine, Inc.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Dassault Systemes SE
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. GNS Healthcare Inc.
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. The AnyLogic Company
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. InSilicoTrials
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Immunetrics Inc.
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Nuventra Pharma Sciences
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Abzena Ltd.
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/39181
Contact Us:
Vision Research Reports
Call: +1 9197 992 333
