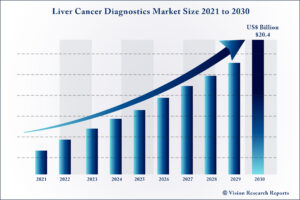
The global Liver Cancer Diagnostics market size is expected to be worth around US$ 20.4 billion by 2030, according to a new report by Vision Research Reports.
The global Liver Cancer Diagnostics market size was valued at US$ 10.7 billion in 2020 and is anticipated to grow at a CAGR of 8.5% during forecast period 2021 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/38898
Table of Contents
Liver Cancer Diagnostics Market Growth Factors
rise in the number of programs to raise awareness about early diagnosis of liver cancer is also expected to be a key factor responsible for increasing the demand for its diagnostic products worldwide. The major risk factors for this disease include chronic viral hepatitis, Cirrhosis, heavy alcohol consumption, tobacco consumption, and obesity. Early screening and diagnosis of liver cancer is the core stone for the control of related mortality, thus, creates a high demand for innovative diagnostics tests. Liver cancer is one of the leading causes of cancer-related deaths around the world. According to the American Cancer Society, in the past few decades, the incidence rates of liver cancer have more than tripled and the death rates have more than doubled during this time.
Liver Cancer Diagnostics Market Report Coverage
| Report Scope | Details |
| Market Size | US$ 20.4 Billion by 2030 |
| Growth Rate | CAGR of 8.5% From 2021 to 2030 |
| Base Year | 2021 |
| Forecast Period | 2021 to 2030 |
| Segments Covered | Test type, End use |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | Abbott Laboratories; F. Hoffmann-La Roche Ltd.; Qiagen N.V.; Thermo Fisher Scientific, Inc.; Siemens Healthineers; Becton Dickinson & Company; Illumina, Inc.; Koninklijke Philips N.V; Epigenomics AG; Fujifilm Medical Systems U.S.A., Inc. |
By Test Type Analysis
the laboratory tests segment dominated the market for liver cancer diagnostics and accounted for the largest revenue share of 39.5%. The segment is projected to grow at the fastest rate during the forecast period. This large share and growth can be attributed to the high preference for laboratory tests due to their accuracy and cost-efficiency.
The imaging tests segment accounted for the second-largest revenue share and is anticipated to show significant growth throughout the study period. Imaging tests can be supportive tests for laboratory and genetic tests that help confirm the presence of a tumor. Imaging tests include Computed Tomography (CT) scan, Magnetic Resonance Imaging (MRI) scan, X-ray, ultrasound, and other radiographic tests.
By End-use Analysis
The hospitals and diagnostic laboratories segment dominated the market for liver cancer diagnostics in 2020 and accounted for the largest revenue share of 83.4%. Hospitals are primary care settings for the diagnosis and treatment of medical conditions including hepatocellular carcinoma.
The pharmaceutical and CRO laboratories segment is anticipated to witness the fastest CAGR during the forecast period. This high growth can be attributed to the high demand for liver diagnostics solutions by leading pharmaceutical companies for research purposes.
By Regional Analysis
North America dominated the liver cancer diagnostics market and accounted for the largest revenue share of 40.1% in 2020 and is projected to remain dominant throughout the forecast period.
In Asia Pacific, the market for liver cancer diagnostics is expected to exhibit the highest growth throughout the study period. This high growth can be attributed to the high prevalence of Hepatitis in emerging countries including India and China.
Read also @ Healthcare Customer Data Platform Market Size, Trends And Forecast To 2030
Major Key Players Covered in The Liver Cancer Diagnostics Market Report include
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd.
- Qiagen N.V.
- Thermo Fisher Scientific, Inc.
- Siemens Healthineers
- Becton Dickinson & Company
- Illumina, Inc.
- Koninklijke Philips N.V.
- Epigenomics AG
- Fujifilm Medical Systems U.S.A., Inc.
Liver Cancer Diagnostics Market Segmentation
- By Test Type
- Laboratory Tests
- Biomarkers
- Oncofetal and Glycoprotein Antigens
- Enzymes and Isoenzymes
- Growth Factors and Receptors
- Molecular Markers
- Pathological Biomarkers
- Blood Tests
- Biomarkers
- Imaging Tests
- Endoscopy
- Biopsy
- Others
- Laboratory Tests
- By End-use
- Hospitals & Diagnostic Laboratories
- Academic & Research Institutes
- Pharmaceutical & CRO Laboratories
- Regional
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- France
- Italy
- Spain
- Russia
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Singapore
- Latin America
- Brazil
- Mexico
- Argentina
- MEA
- South Africa
- Saudi Arabia
- UAE
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Liver Cancer Diagnostics Market Snapshot
Chapter 4. Liver Cancer Diagnostics Market Variables and Scope
4.1. Introduction
4.2. Liver Cancer Diagnostics Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Liver Cancer Diagnostics Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Liver Cancer Diagnostics Market, By Test Type
7.1. Liver Cancer Diagnostics Market, by Test Type, 2021-2030
7.1.1. Laboratory Tests
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Imaging Tests
7.1.2.1. Market Revenue and Forecast (2017-2030)
7.1.3. Endoscopy
7.1.3.1. Market Revenue and Forecast (2017-2030)
7.1.4. Biopsy
7.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Liver Cancer Diagnostics Market, By End-use
8.1. Liver Cancer Diagnostics Market, by End-use, 2021-2030
8.1.1. Hospitals & Diagnostic Laboratories
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Academic & Research Institutes
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Pharmaceutical & CRO Laboratories
8.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Liver Cancer Diagnostics Market, Regional Estimates and Trend Forecast
9.1. North America
9.1.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.1.2. Market Revenue and Forecast, by End-use (2017-2030)
9.1.3. U.S.
9.1.3.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.1.3.2. Market Revenue and Forecast, by End-use (2017-2030)
9.1.4. Rest of North America
9.1.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.1.4.2. Market Revenue and Forecast, by End-use (2017-2030)
9.2. Europe
9.2.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.2.2. Market Revenue and Forecast, by End-use (2017-2030)
9.2.3. UK
9.2.3.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.2.3.2. Market Revenue and Forecast, by End-use (2017-2030)
9.2.4. Germany
9.2.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.2.4.2. Market Revenue and Forecast, by End-use (2017-2030)
9.2.5. France
9.2.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.2.5.2. Market Revenue and Forecast, by End-use (2017-2030)
9.2.6. Rest of Europe
9.2.6.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.2.6.2. Market Revenue and Forecast, by End-use (2017-2030)
9.3. APAC
9.3.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.3.2. Market Revenue and Forecast, by End-use (2017-2030)
9.3.3. India
9.3.3.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.3.3.2. Market Revenue and Forecast, by End-use (2017-2030)
9.3.4. China
9.3.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.3.4.2. Market Revenue and Forecast, by End-use (2017-2030)
9.3.5. Japan
9.3.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.3.5.2. Market Revenue and Forecast, by End-use (2017-2030)
9.3.6. Rest of APAC
9.3.6.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.3.6.2. Market Revenue and Forecast, by End-use (2017-2030)
9.4. MEA
9.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.4.2. Market Revenue and Forecast, by End-use (2017-2030)
9.4.3. GCC
9.4.3.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.4.3.2. Market Revenue and Forecast, by End-use (2017-2030)
9.4.4. North Africa
9.4.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.4.4.2. Market Revenue and Forecast, by End-use (2017-2030)
9.4.5. South Africa
9.4.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.4.5.2. Market Revenue and Forecast, by End-use (2017-2030)
9.4.6. Rest of MEA
9.4.6.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.4.6.2. Market Revenue and Forecast, by End-use (2017-2030)
9.5. Latin America
9.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.5.2. Market Revenue and Forecast, by End-use (2017-2030)
9.5.3. Brazil
9.5.3.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.5.3.2. Market Revenue and Forecast, by End-use (2017-2030)
9.5.4. Rest of LATAM
9.5.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
9.5.4.2. Market Revenue and Forecast, by End-use (2017-2030)
Chapter 10. Company Profiles
10.1. Abbott Laboratories
10.1.1. Company Overview
10.1.2. Test Type Offerings
10.1.3. Financial Performance
10.1.4. Recent Initiatives
10.2. F. Hoffmann-La Roche Ltd.
10.2.1. Company Overview
10.2.2. Test Type Offerings
10.2.3. Financial Performance
10.2.4. Recent Initiatives
10.3. Qiagen N.V.
10.3.1. Company Overview
10.3.2. Test Type Offerings
10.3.3. Financial Performance
10.3.4. Recent Initiatives
10.4. Thermo Fisher Scientific, Inc.
10.4.1. Company Overview
10.4.2. Test Type Offerings
10.4.3. Financial Performance
10.4.4. Recent Initiatives
10.5. Siemens Healthineers
10.5.1. Company Overview
10.5.2. Test Type Offerings
10.5.3. Financial Performance
10.5.4. Recent Initiatives
10.6. Becton Dickinson & Company
10.6.1. Company Overview
10.6.2. Test Type Offerings
10.6.3. Financial Performance
10.6.4. Recent Initiatives
10.7. Illumina, Inc.
10.7.1. Company Overview
10.7.2. Test Type Offerings
10.7.3. Financial Performance
10.7.4. Recent Initiatives
10.8. Koninklijke Philips N.V.
10.8.1. Company Overview
10.8.2. Test Type Offerings
10.8.3. Financial Performance
10.8.4. Recent Initiatives
10.9. Epigenomics AG
10.9.1. Company Overview
10.9.2. Test Type Offerings
10.9.3. Financial Performance
10.9.4. Recent Initiatives
10.10. Fujifilm Medical Systems U.S.A., Inc.
10.10.1. Company Overview
10.10.2. Test Type Offerings
10.10.3. Financial Performance
10.10.4. Recent Initiatives
Chapter 11. Research Methodology
11.1. Primary Research
11.2. Secondary Research
11.3. Assumptions
Chapter 12. Appendix
12.1. About Us
12.2. Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/38898
Contact Us:
Vision Research Reports
Call: +1 9197 992 333
