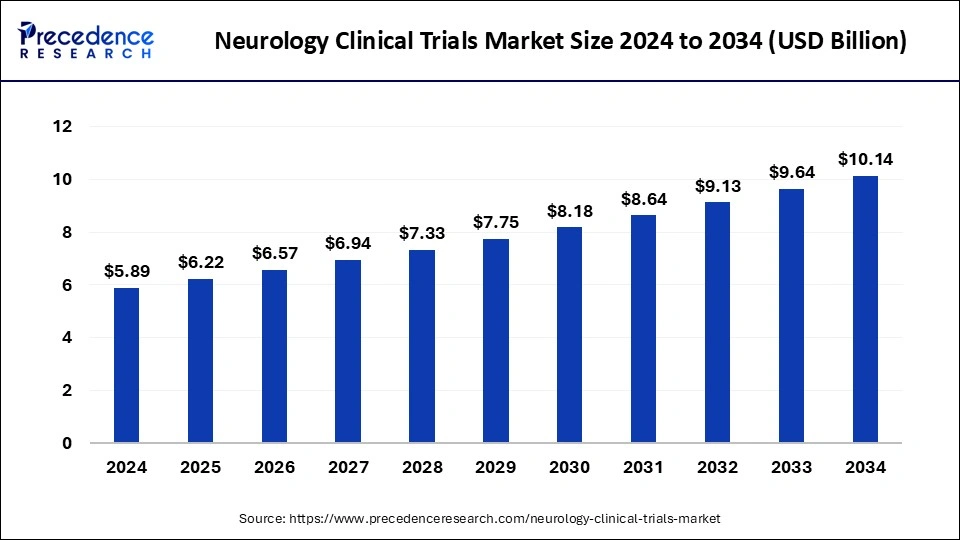
The global neurology clinical trials market size was valued at USD 5.58 billion in 2023 and is predicted to reach around USD 9.64 billion by 2033, at a CAGR of 5.62% from 2024 to 2033.
Neurology clinical trials focus on researching and developing new treatments for neurological disorders, which include conditions such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, stroke, and amyotrophic lateral sclerosis (ALS). The market for these trials has been expanding due to the rising prevalence of neurological disorders, increasing awareness about mental health, and advancements in medical technology. The demand for innovative treatments and the high burden of neurological diseases are driving the growth of this market.
The global neurology clinical trials market is segmented by phase (Phase I, Phase II, Phase III, and Phase IV), study design (interventional, observational, and expanded access), and indication (Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, stroke, ALS, and others). The interventional segment holds a significant share due to the extensive use of randomized controlled trials (RCTs) in assessing the efficacy and safety of new treatments.
Pharmaceutical and biotechnology companies are the major sponsors of these trials, but academic institutions and government organizations also play a critical role. The involvement of various stakeholders ensures a comprehensive approach to tackling neurological diseases, contributing to the market’s robustness.
Advancements in Technology
- Digital Health Technologies: The integration of digital health technologies, such as wearable devices and mobile health apps, is enhancing the monitoring and data collection in neurology clinical trials.
- Artificial Intelligence (AI) and Machine Learning (ML): AI and ML are being utilized to identify patterns in neurological diseases, optimize trial design, and predict patient outcomes, leading to more efficient and effective trials.
Increased Focus on Personalized Medicine
- Genomics and Biomarkers: The use of genomics and biomarkers in clinical trials is enabling a more personalized approach to treating neurological disorders. This trend is driving the development of targeted therapies and improving trial outcomes.
- Stratified Patient Populations: Trials are increasingly stratifying patient populations based on genetic, biomarker, and phenotypic characteristics to identify subgroups that respond better to specific treatments.
Growth in Neurodegenerative Disease Research
- Alzheimer’s and Parkinson’s Disease: There is a growing number of clinical trials focused on neurodegenerative diseases such as Alzheimer’s and Parkinson’s. This is driven by the aging population and the urgent need for effective treatments.
- Innovative Therapeutics: Development of novel therapeutics, including monoclonal antibodies, gene therapies, and small molecules, is a key trend in addressing neurodegenerative conditions.
Get a Sample: https://www.precedenceresearch.com/sample/4657
Regional Insights
North America, particularly the United States, dominates the neurology clinical trials market due to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and significant government funding for research. The region’s robust regulatory framework and high investment in R&D also support the market. Additionally, the high prevalence of neurological disorders and the growing geriatric population further boost the demand for clinical trials in neurology.
Europe is another major market for neurology clinical trials, driven by countries such as Germany, the UK, and France. The European market benefits from strong healthcare systems, significant research funding, and collaborations between pharmaceutical companies and research institutions. The European Union’s support for research through programs like Horizon 2020 also plays a crucial role in advancing neurological research. The Asia-Pacific region is experiencing rapid growth in the neurology clinical trials market due to the increasing prevalence of neurological disorders, improving healthcare infrastructure, and rising investment in medical research. Countries like China, Japan, South Korea, and India are at the forefront of this growth. The region’s large and diverse patient population offers significant opportunities for conducting clinical trials, making it an attractive destination for global pharmaceutical companies.
Neurology Clinical Trials Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 9.64 Billion |
| Market Size in 2023 | USD 5.58 Billion |
| Market Size in 2024 | USD 5.89 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 5.62% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Phase, Study Design, Indication, Study Design, Phase, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Neurology Clinical Trials Market Dynamics
Drivers
Rising Prevalence of Neurological Disorders: The increasing incidence of neurological disorders is one of the primary drivers of the neurology clinical trials market. Conditions such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and epilepsy are becoming more prevalent due to aging populations and lifestyle changes. This rise in neurological disorders necessitates the development of new treatments, fueling the demand for clinical trials.
Advancements in Medical Technology: Technological advancements in imaging techniques, biomarkers, and genetic testing have revolutionized the field of neurology. These innovations enable better diagnosis, monitoring, and understanding of neurological disorders, facilitating the design and execution of clinical trials. Improved technology also enhances patient recruitment and data collection, making trials more efficient.
Government and Private Funding: Significant funding from government bodies, non-profit organizations, and private companies supports neurological research and clinical trials. Programs like the National Institutes of Health (NIH) in the US and the European Union’s Horizon 2020 provide substantial grants for neurological research. This funding is crucial for conducting extensive clinical trials and developing new treatments.
Growing Awareness and Patient Advocacy: Increased awareness about neurological disorders and the importance of clinical trials has led to greater patient participation and advocacy. Patient advocacy groups play a vital role in educating patients and their families about clinical trials, encouraging participation, and ensuring patient-centric trial designs. This increased involvement of patients boosts the market for neurology clinical trials.
Opportunities
Emerging Markets: Emerging markets in Asia-Pacific, Latin America, and the Middle East offer significant growth opportunities for the neurology clinical trials market. These regions have large patient populations, improving healthcare infrastructure, and increasing investments in medical research. Conducting clinical trials in these regions can provide valuable data on diverse patient populations and enhance the generalizability of trial results.
Collaborative Research: Collaborations between pharmaceutical companies, academic institutions, and research organizations can drive innovation in neurological research. Collaborative efforts enable sharing of resources, expertise, and data, leading to more comprehensive and efficient clinical trials. Public-private partnerships also play a crucial role in advancing research and developing new treatments.
Personalized Medicine: The trend towards personalized medicine presents opportunities for neurology clinical trials. Understanding the genetic and molecular basis of neurological disorders allows for the development of targeted therapies tailored to individual patients. Personalized approaches can improve treatment outcomes and reduce adverse effects, making clinical trials more effective and relevant.
Innovative Trial Designs: The adoption of innovative trial designs, such as adaptive trials and decentralized trials, can enhance the efficiency and flexibility of neurology clinical trials. Adaptive trials allow modifications based on interim results, reducing the time and cost of trials. Decentralized trials leverage digital technologies to conduct trials remotely, increasing patient accessibility and engagement.
Challenges
High Costs and Complexity: Neurology clinical trials are often expensive and complex due to the intricate nature of neurological disorders and the need for specialized equipment and expertise. The high costs associated with conducting these trials can be a significant barrier, especially for smaller companies and academic institutions. Securing adequate funding and resources is a major challenge in this market.
Regulatory Hurdles: The stringent regulatory requirements for clinical trials can pose challenges for sponsors. Ensuring compliance with regulations, obtaining necessary approvals, and adhering to ethical standards require significant time and effort. Regulatory variations across regions further complicate the process, making it essential for sponsors to navigate diverse regulatory landscapes effectively.
Patient Recruitment and Retention: Recruiting and retaining patients for neurology clinical trials can be challenging due to various factors, including disease severity, patient mobility, and caregiver involvement. Ensuring sufficient patient enrollment and minimizing dropouts are critical for the success of clinical trials. Strategies to improve patient recruitment and retention, such as patient-centric trial designs and effective communication, are essential.
Ethical Considerations: Neurology clinical trials must address ethical considerations, particularly when involving vulnerable populations such as patients with cognitive impairments. Ensuring informed consent, protecting patient privacy, and maintaining ethical standards are paramount. Balancing the need for scientific advancement with ethical responsibilities requires careful planning and oversight.
Data Management and Analysis: The large volumes of data generated in neurology clinical trials necessitate robust data management and analysis systems. Ensuring data accuracy, security, and compliance with regulatory requirements is crucial. Advanced data analytics and artificial intelligence (AI) tools can aid in managing and interpreting complex data sets, but their implementation requires significant investment and expertise.
Read Also: Menstrual Health Apps Market Size to Rise USD 9.04 Bn by 2033
Recent Developments
- In August 2023, the phase II RECOVER-NEURO clinical trial study to evaluate the combination of REMOTE-transcranial direct current stimulation (tDCS) and a brain training program for long covid was launched by Soterix Medical.
- In March 2024, an innovative and new pTau217 blood test, ALZpath Dx, was launched by a specialized clinical laboratory, Neurocode USA, Inc., that offers world-class testing solutions for neurological disorders. This new test may be used in the monitoring, screening, and diagnosis of Alzheimer’s disease. In the US, Neurocode is the first laboratory to make this test as LDT (laboratory-developed test) for clinical trials, clinical diagnostics use, and other research causes.
Neurology Clinical Trials Market Companies
- IQVIA
- Biogen
- Aurora Healthcare
- GlaxoSmithKline Plc.
- Icon Plc.
- Syneous Health
- Charles River Laboratories
- Med pace
- Covance
- Novartis AG
- Sanofi
- Merck & Co., Inc.
- AbbVie Inc.
- Teva Pharmaceutical Industries Ltd.
- Annovis Bio
- Athira Pharma, Inc.
- Zydus Group
- Eli Lilly and Company
- Eisai Co., Ltd.
- AstraZeneca
- Supernus Pharmaceuticals, Inc. (Adamas Pharmaceuticals)
Segments Covered in the Report
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- Epilepsy
- Parkinson’s Disease (PD)
- Huntington’s Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Others
By Study Design
- Epilepsy
- Interventional
- Observational
- Expanded Access
- Parkinson’s Disease (PD)
- Interventional
- Observational
- Expanded Access
- Huntington’s Disease
- Interventional
- Observational
- Expanded Access
- Stroke
- Interventional
- Observational
- Expanded Access
- Traumatic Brain Injury (TBI)
- Interventional
- Observational
- Expanded Access
- Amyotrophic Lateral Sclerosis (ALS)
- Interventional
- Observational
- Expanded Access
- Muscle Regeneration
- Interventional
- Observational
- Expanded Access
- Others
- Interventional
- Observational
- Expanded Access
by Phase
- Epilepsy
- Phase I
- Phase II
- Phase III
- Phase IV
- Parkinson’s Disease (PD)
- Phase I
- Phase II
- Phase III
- Phase IV
- Huntington’s Disease
- Phase I
- Phase II
- Phase III
- Phase IV
- Stroke
- Phase I
- Phase II
- Phase III
- Phase IV
- Traumatic Brain Injury (TBI)
- Phase I
- Phase II
- Phase III
- Phase IV
- Amyotrophic Lateral Sclerosis (ALS)
- Phase I
- Phase II
- Phase III
- Phase IV
- Muscle Regeneration
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
