Table of Contents
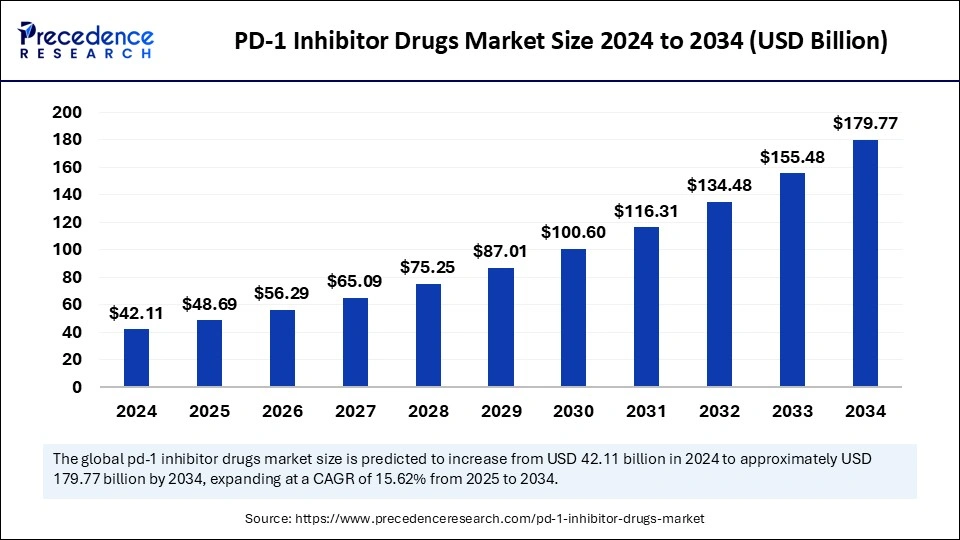
The global PD-1 inhibitor drugs market size was valued at USD 42.11 billion in 2024 and is projected to reach around USD 179.77 billion by 2034, growing at a CAGR of 15.62%. The market is driven by rising cancer incidence, expanding regulatory approvals, advanced combination therapeutic approaches, and improved accessibility to innovative immunotherapy.
Get Sample Copy of Report@ https://www.precedenceresearch.com/sample/5718
PD-1 Inhibitor Drugs Market Key Points
- North America held the largest share of the PD-1 inhibitor drugs market in 2024.
- Asia Pacific is projected to experience the fastest growth in the coming years.
- The pembrolizumab segment led the market by drug type in 2024.
- The nivolumab segment is expected to expand significantly over the forecast period.
- The non-small cell lung cancer segment dominated the market in 2024.
- The melanoma segment is forecasted to grow substantially during the projected period.
- The hospital pharmacies segment held the highest market share by distribution channel in 2024.
- The online pharmacies segment is anticipated to witness notable growth over the forecast period.
Also Read: Computational Biology Market
AI Impact on PD-1 Inhibitor Drugs Market
1. Drug Discovery and Development
AI technologies facilitate the identification and optimization of new PD-1 inhibitors by analyzing vast datasets to predict drug efficacy and safety. This accelerates the drug discovery process, allowing for faster development of novel therapies that can enhance patient outcomes in cancer treatment.
2. Personalized Medicine
AI plays a crucial role in the shift towards personalized medicine within the PD-1 inhibitor market. By leveraging machine learning algorithms, healthcare providers can analyze genetic profiles and biomarker expressions to tailor treatments to individual patients. This customization improves therapeutic efficacy and minimizes adverse effects, leading to better patient management.
3. Clinical Trials Optimization
AI enhances the efficiency of clinical trials for PD-1 inhibitors by optimizing patient selection and monitoring. Predictive analytics can identify suitable candidates for trials based on genetic markers and disease characteristics, thereby improving trial success rates and reducing costs associated with failed studies.
4. Market Growth and Expansion
The integration of AI in the PD-1 inhibitor landscape supports market expansion by driving innovation and improving treatment outcomes. The global PD-1 inhibitor drugs market is projected to grow from USD 41.4 billion in 2024 to USD 201.5 billion by 2035, fueled by rising cancer prevalence and advancements in immunotherapy applications1. AI’s capabilities in enhancing drug efficacy and streamlining processes contribute significantly to this growth trajectory.
5. Combination Therapies
AI is instrumental in exploring combination therapies involving PD-1 inhibitors with other treatments such as chemotherapy or targeted therapies. By analyzing clinical data, AI can identify synergistic effects that improve patient survival rates, further increasing the acceptance and utilization of PD-1 inhibitors in clinical practice.
Uses of PD-1 Inhibitor Drugs
-
Treatment of Non-Small Cell Lung Cancer (NSCLC)
- PD-1 inhibitors like pembrolizumab and nivolumab are widely used in treating NSCLC by enhancing the immune system’s ability to target and destroy cancer cells.
-
Management of Melanoma
- These drugs help treat advanced melanoma by blocking the PD-1 pathway, allowing immune cells to attack melanoma tumors more effectively.
-
Therapy for Head and Neck Cancers
- PD-1 inhibitors are approved for treating recurrent or metastatic head and neck squamous cell carcinoma, improving survival rates and reducing tumor progression.
-
Treatment of Hodgkin Lymphoma
- PD-1 inhibitors are effective in treating relapsed or refractory classical Hodgkin lymphoma by boosting immune response against lymphoma cells.
-
Bladder Cancer Therapy
- Patients with advanced or metastatic urothelial carcinoma benefit from PD-1 inhibitors when chemotherapy fails, offering an alternative treatment option.
-
Colorectal Cancer with MSI-H or dMMR
- PD-1 inhibitors are used in treating metastatic colorectal cancer in patients with high microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR).
-
Renal Cell Carcinoma (Kidney Cancer)
- PD-1 inhibitors help slow the progression of advanced kidney cancer, often used alone or in combination with other targeted therapies.
-
Triple-Negative Breast Cancer (TNBC)
- Certain PD-1 inhibitors are approved for treating advanced or metastatic TNBC in combination with chemotherapy, enhancing immune response against tumor cells.
-
Gastric and Esophageal Cancers
- PD-1 inhibitors are used to treat advanced gastric and esophageal cancers, particularly in cases where standard treatments are ineffective.
-
Hepatocellular Carcinoma (Liver Cancer)
- These drugs provide an alternative treatment for patients with liver cancer who do not respond well to traditional therapies, improving survival rates.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 179.77 Billion |
| Market Size in 2025 | USD 48.69 Billion |
| Market Size in 2024 | USD 42.11 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 15.62% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Drug Type, Indication, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Market Drivers
The increasing incidence of cancers, such as non-small cell lung cancer (NSCLC) and melanoma, is a major driver of market growth. Growing investments in immunotherapy research, coupled with expanding clinical trials, are further propelling the market. Additionally, pharmaceutical companies are investing in novel drug formulations and seeking regulatory approvals for expanded indications, which contributes to market expansion.
Market Opportunities
There is significant potential for PD-1 inhibitors in combination therapies, where they are used alongside chemotherapy, targeted therapies, or other immune checkpoint inhibitors to enhance treatment efficacy. Emerging markets, particularly in Asia Pacific and Latin America, offer lucrative opportunities due to increasing access to healthcare and favorable regulatory changes. Research into PD-1 inhibitors for non-oncology applications, such as infectious diseases and autoimmune disorders, presents another avenue for growth.
Market Challenges
Challenges facing the PD-1 inhibitor drugs market include high treatment costs, accessibility issues in developing regions, and regulatory complexities surrounding immunotherapies. The potential for immune-related adverse effects and therapy resistance also poses concerns for both patients and healthcare providers. Moreover, competition from alternative immunotherapies, such as PD-L1 inhibitors and novel cancer treatment modalities, presents an additional challenge.
Regional Outlook
The North American market is thriving due to strong government support, reimbursement policies, and a high adoption rate of PD-1 inhibitors. Europe remains a key player, driven by advanced cancer research and growing acceptance of immunotherapy. Meanwhile, Asia Pacific is emerging as a high-growth region, fueled by rising cancer prevalence, increasing clinical trial activities, and enhanced accessibility to immunotherapy drugs.
PD-1 inhibitor drugs market Market Companies

- Akeso Inc.
- Alphamab Oncology
- Amgen Inc.
- AstraZeneca Plc
- BeiGene Ltd.
- Bristol Myers Squibb Co.
- Chia Tai Tianqing Pharmaceutical Group Co. Ltd.
- Eli Lilly and Co.
- F. Hoffmann La Roche Ltd.
- Gilead Sciences Inc.
- GlaxoSmithKline Plc
- Innovent Biologics Inc.
- Jiangsu Hengrui Pharmaceuticals Co. Ltd.
- Merck and Co. Inc.
Recent Developments
- In November 2024, Dr. Reddy’s Laboratories initiated Zytorvi (Toripalimab) sales as a next-generation immune therapy for patients suffering from head and neck cancer and nasopharyngeal carcinoma. This next-gen PD-1 inhibitor makes India the world’s third nation to access the treatment, which presents a better medical alternative than standard chemotherapy.
- In September 2024, the FDA granted approval to Roche for Tecentriq Hybreza (atezolizumab and hyaluronidase-tqjs) as a first PD-L1 inhibitor with subcutaneous administration access. Approval permits Tecentriq Hybreza for treating every adult disease indication of intravenous Tecentriq among selected lung cancer and skin cancer types with liver and soft tissue types.
Segments Covered in the Report
By Drug Type
- Pembrolizumab
- Nivolumab
- Cemiplimab
- Dostarlimab
- Others
By Indication
- Melanoma
- Hodgkin Lymphoma
- Non-Small Cell Lung Cancer
- Kidney Cancer
- Head And Neck Cancers
- Stomach Cancer
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Geography
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/