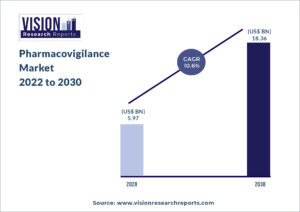
The global pharmacovigilance market size was valued at USD 5.97 billion in 2020, and is predicted to be worth around USD 18.36 billion by 2030, registering a CAGR of 10.6% during the forecast period 2022 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/39099
Table of Contents
Pharmacovigilance Market Growth Factors
Increasing incidence of Adverse Drug Reactions (ADRs) is the key growth driver. ADR imposes a substantial burden on healthcare systems and is one of the prominent causes of morbidity in developed countries. According to the National Center for Biotechnology Information (NCBI), approximately 5% of total hospitalizations in Europe each year are due to ADR. Pharmacovigilance services play an integral role in this clinical trial phase by assisting manufacturers in identifying adverse effects associated with the drug.
An increase in the prevalence of chronic diseases such as oncological diseases, diabetes, and cardiovascular and respiratory disorders has led to an increase in drug consumption worldwide. Therefore, the demand for new drug development via extensive clinical trials has increased. Pharmacovigilance (PV) is the inevitable part of drug discovery and development procedures.
Pharmacovigilance Market Report Coverage
| Report Scope | Details |
| Market Size | US$ 18.36 billion by 2030 |
| Growth Rate | CAGR of 10.6% From 2022 to 2030 |
| Largest Market | North America |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Service Provider, Product life cycle, Type, Process Flow, Therapeutic area, End-use |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | Accenture; Linical Accelovance; Cognizant; Laboratory Corporation of America Holdings; IBM Corporation; ArisGlobal; ICON plc.; Capgemini; ITClinical; FMD K&L; IQVIA; TAKE Solutions Ltd.; PAREXEL International Corporation; BioClinica Inc.; Wipro Ltd.; United BioSource Corporation |
By Service Provider Analysis
Contract outsourcing held the largest share of over 55.0% in 2021 and is expected to witness the fastest growth in the forthcoming years. The growth can be attributed to the benefits associated with outsourcing such as risk mitigation, resource flexibility, reduction of upfront investments, and lower fixed cost.
The dynamic growth of the contract outsourcing segment can also be attributed to the rapidly emerging CROs providing end-to-end clinical trial solutions, especially in the emerging economies of Asia Pacific, such as India, China, and Japan, enabling resources sharing, cost efficiency, resource flexibility, and the expansion of operative capabilities. Contract outsourcing also helps reduce the complexity of clinical trials, allows faster approval of trials, and helps effective utilization of internal resources.
By Product Life Cycle Analysis
The phase IV (post-marketing) segment led the overall market with a revenue share of over 75.0% in 2021. These solutions act as an additional safety measure for the drugs undergoing clinical trials.
The phase III segment is expected to witness lucrative growth over the forecast period. Phase III trials are done to determine and establish the efficacy of drugs. These trials also provide additional information regarding possible drug interactions, drug safety, and effectiveness before the commercialization of the drug.
By Type Analysis
Spontaneous reporting held the largest share of over 30.0% in 2021 owing to wide usage in the detection of new, serious, and rare ADRs and serves as an efficient and inexpensive method.
Cohort Event Monitoring (CEM) emerged as the second-largest segment in 2021 owing to the increasing application in the detection of a wide range of adverse clinical events. Conjugation of CEM with statistical tools and data mining systems such as longitudinal health records are responsible for the growing popularity of this type.
Targeted spontaneous reporting is projected to be the fastest-growing segment over the forecast period owing to the rising government initiatives to incorporate reporting methodologies other than spontaneous reporting by the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP).
Electronic Health Record (EHR) mining is increasingly used to identify risk factors for patients after discharge from hospitals. Electronic health records are imperative sources of medical information about clinical events in hospitals and research organizations.
By Therapeutic Area Analysis
The oncology segment held the largest revenue share of over 25.0% in 2021. Monitoring the safety of cancer drugs is very important due to the associated side effects, which is propelling the demand for pharmacovigilance services.
Pharmacovigilance helps in the early detection and spontaneous reporting of adverse drug reactions. Moreover, recent advancements in cancer treatments, such as targeted therapy, have some serious adverse effects and can compromise a patient’s quality of life.
By Process Flow Analysis
Signal detection dominated the market with a revenue share of over 35.0% in 2021. Spontaneous Reporting Systems (SRSs) use the dominant source of signals through which the suspected cases get voluntarily reported by the healthcare professionals to the other regulatory bodies.
Artificial Intelligence (AI) and big data are being used by companies for better assessment of signals. The case data management segment is expected to exhibit the fastest growth rate over the forecast period.
By End-use Analysis
Pharmaceuticals held the largest revenue share of over 40.0% in terms of revenue. Outsourcing the pharmacovigilance process is practiced by pharma companies to avoid high upfront investments and fixed overhead costs, increase resource flexibility, and secure additional capacity.
The biotechnology segment is anticipated to witness lucrative growth in the forthcoming years owing to increasing new product development activities in this sector. In recent years, drugs are being developed and consumed at increasingly high rates.
By Regional Analysis
North America held the largest revenue share of over 30.0% in 2021 owing to the presence of key pharmaceutical and medical devices players, contributing to the overall revenue in this region.
Asia Pacific is expected to register the fastest CAGR of 12.1% during the forecast period owing to the availability of various outsourcing organizations. Consequentially, there is improved productivity, cost efficiency, and resource sharing that is anticipated to propel the regional demand for pharmacovigilance in the forthcoming years.
Read also @ Medical Fiber Optics Market to Expand at a CAGR of 6.02% from 2021 to 2030
Major Key Players Covered in The Pharmacovigilance Market Report include
- Accenture
- Linical Accelovance
- Cognizant
- Laboratory Corporation of America Holdings
- IBM Corporation
- ArisGlobal
- ICON plc.
- Capgemini
- ITClinical
- FMD K&L
- IQVIA
- TAKE Solutions Ltd.
- PAREXEL International Corporation
- BioClinica Inc.
- Wipro Ltd.
- United BioSource Corporation
Pharmacovigilance Market Segmentation
- By Service Provider
- In-house
- Contract Outsourcing
- By Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- By Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- By Process Flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- By End-use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Regional
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Russia
- Asia Pacific
- Japan
- China
- India
- Latin America
- Brazil
- Mexico
- Middle East and Africa
- South Africa
- Kingdom of Saudi Arabia
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Pharmacovigilance Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Pharmacovigilance Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Pharmacovigilance Market, By Service Provider
7.1. Pharmacovigilance Market, by Service Provider, 2021-2030
7.1.1. In-house
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Contract Outsourcing
7.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Pharmacovigilance Market, By Product Life Cycle
8.1. Pharmacovigilance Market, by Product Life Cycle, 2021-2030
8.1.1. Pre-clinical
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Phase I
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Phase II
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Phase III
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Phase IV
8.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Pharmacovigilance Market, By Type
9.1. Pharmacovigilance Market, by Type, 2021-2030
9.1.1. Spontaneous Reporting
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Intensified ADR Reporting
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Targeted Spontaneous Reporting
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Cohort Event Monitoring
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. EHR Mining
9.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Pharmacovigilance Market, By Process Flow
10.1. Pharmacovigilance Market, by Process Flow, 2021-2030
10.1.1. Case Data Management
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Signal Detection
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Risk Management System
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Pharmacovigilance Market, By Therapeutic Area
11.1. Pharmacovigilance Market, by Therapeutic Area, 2021-2030
11.1.1. Oncology
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Neurology
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Cardiology
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Respiratory Systems
11.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Pharmacovigilance Market, By End-use
12.1. Pharmacovigilance Market, by End-use, 2021-2030
12.1.1. Pharmaceuticals
12.1.1.1. Market Revenue and Forecast (2017-2030)
12.1.2. Biotechnology Companies
12.1.2.1. Market Revenue and Forecast (2017-2030)
12.1.3. Medical Device Manufacturers
12.1.3.1. Market Revenue and Forecast (2017-2030)
12.1.4. Others
12.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 13. Global Pharmacovigilance Market, Regional Estimates and Trend Forecast
13.1. North America
13.1.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.1.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.1.3. Market Revenue and Forecast, by Type (2017-2030)
13.1.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.1.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.1.6. Market Revenue and Forecast, by End-use (2017-2030)
13.1.7. U.S.
13.1.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.1.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.1.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.1.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.1.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.1.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.1.8. Rest of North America
13.1.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.1.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.1.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.1.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.1.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.1.8.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2. Europe
13.2.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2.7. UK
13.2.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2.8. Germany
13.2.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.8.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2.9. France
13.2.9.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.9.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.9.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.9.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.9.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.9.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2.10. Rest of Europe
13.2.10.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.10.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.10.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.10.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.10.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.10.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3. APAC
13.3.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3.7. India
13.3.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3.8. China
13.3.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.8.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3.9. Japan
13.3.9.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.9.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.9.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.9.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.9.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.9.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3.10. Rest of APAC
13.3.10.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.10.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.10.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.10.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.10.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.10.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4. MEA
13.4.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4.7. GCC
13.4.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4.8. North Africa
13.4.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.8.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4.9. South Africa
13.4.9.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.9.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.9.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.9.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.9.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.9.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4.10. Rest of MEA
13.4.10.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.10.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.10.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.10.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.10.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.10.6. Market Revenue and Forecast, by End-use (2017-2030)
13.5. Latin America
13.5.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.5.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.5.3. Market Revenue and Forecast, by Type (2017-2030)
13.5.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.5.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.5.6. Market Revenue and Forecast, by End-use (2017-2030)
13.5.7. Brazil
13.5.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.5.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.5.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.5.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.5.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.5.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.5.8. Rest of LATAM
13.5.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.5.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.5.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.5.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.5.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.5.8.6. Market Revenue and Forecast, by End-use (2017-2030)
Chapter 14. Company Profiles
14.1. Accenture
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. Linical Accelovance
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. Cognizant
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. Laboratory Corporation of America Holdings
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. IBM Corporation
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. ArisGlobal
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. ICON plc.
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. Capgemini
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
14.9. ITClinical
14.9.1. Company Overview
14.9.2. Product Offerings
14.9.3. Financial Performance
14.9.4. Recent Initiatives
14.10. FMD K&L
14.10.1. Company Overview
14.10.2. Product Offerings
14.10.3. Financial Performance
14.10.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. Appendix
16.1. About Us
Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/39099
Contact Us:
Vision Research Reports
Call: +1 9197 992 333