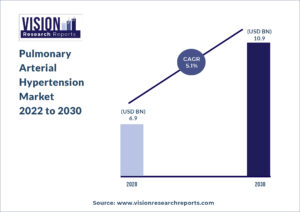
The global pulmonary arterial hypertension market size was valued at USD 6.9 billion in 2020, and is predicted to be worth around USD 10.9 billion by 2030, registering a CAGR of 5.1% during the forecast period 2022 to 2030.
Download Exclusive Sample of Report@ https://www.visionresearchreports.com/report/sample/39125
Table of Contents
Pulmonary Arterial Hypertension Market Growth Factors
Growing initiatives by key market players, high prevalence of pulmonary arterial hypertension, adoption rate, availability of reimbursement, and entry of generics are some of the key drivers of this market. As per the American Lung Association, about 500 to 1000 new PAH patients are being diagnosed every year in the U.S.
The key factors driving the market growth include the increasing prevalence of pulmonary arterial hypertension, drug development and technological advancements, product approvals, and initiatives by key companies.
The COVID-19 pandemic had a notable impact on the market. The pandemic created uncertain market conditions and lead to dampened growth. Other impacts of the pandemic include operational challenges, supply chain bottlenecks, challenges in conducting clinical trials, among others. Market players also reported a reduction in new patient starts and new patient prescriptions during 2020.
Report Coverage
| Report Scope | Details |
| Market Size | US$ 10.9 billion by 2030 |
| Growth Rate | CAGR of 5.1% From 2022 to 2030 |
| Largest Market | North America |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Drug Class, Type, Route of administration |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | United Therapeutics Corporation; Bayer; Gilead Sciences, Inc.; Johnson & Johnson; Viatris Inc.; GlaxoSmithKline; Sandoz Inc. (Novartis); Lupin Pharmaceuticals, Inc.; Sun Pharmaceutical Industries, Inc.; Teva Pharmaceuticals Inc |
By Drug Class Analysis
The prostacyclin and prostacyclin analogs segment dominated the market and accounted for the largest revenue share of around 47.0% in 2021.
The key factors contributing to the large share include a high demand and growing indications. In July 2021, Uptravi belonging to Prostacyclin and Prostacyclin Analogs segment received FDA approval for intravenous use in pulmonary arterial hypertension patients.
The SGC simulators segment is expected to grow at the fastest CAGR of over 5%. sGC stimulators can ensure maximum activation of sGC by potentiating NO-sGC signaling.
By Type Analysis
The branded segment dominated the market and accounted for the largest revenue share of 88.2% in 2021, while the generics segment is anticipated to witness the fastest CAGR of 5.9% in the coming years.
The factors contributing to the growth include the expiry of key patents, the rising launch of generics, growing consumption in developing markets, and initiatives by major companies.
Bayer on the other hand reported a significant increase in revenue of its branded PAH product- Adempas, driven by higher volume sales in the U.S. both in 2020 and 2021. However, the active ingredient patent of Adempas is set to expire in several key markets during the forecast period which may dampen sales in the coming years.
By Route of Administration Analysis
The oral segment dominated the market and held the largest revenue share of over 55.0% in 2021. This is attributable to the growing availability of oral formulations for pulmonary arterial hypertension and patient preference for the oral route of administration.
Letairis, Opsumit, Adcirca, and Revatio are some examples of oral PAH drugs. Johnson & Johnson’s Opsumit tablets, for instance, recorded total sales of USD 1.6 billion in 2020. The sales represented a 23.5% Y-o-Y growth compared to 2019.
The intravenous/ subcutaneous segment is anticipated to register the fastest growth of over 5.0% over the forecast period due to increasing indications and approvals by regulatory agencies and advances in drug delivery systems to increase patient compliance.
By Regional Analysis
North America dominated the pulmonary arterial hypertension market and held the largest revenue share of over 42.0% in 2021. The large share is due to the developed healthcare infrastructure in the U.S. and Canada that facilitates access to advanced therapeutics.
In the Asia Pacific, the market is estimated to witness the fastest CAGR of more than 6.0% over the next few years. This is owing to the growing consumption of generics, the presence of key pharmaceutical companies, and developing healthcare infrastructure.
Read also @ Exoskeleton Market Revenue To Cross USD 1.2 Bn by 2030
Major Key Players Covered in The Pulmonary Arterial Hypertension Market Report include
- United Therapeutics Corporation
- Bayer
- Gilead Sciences, Inc.
- Johnson & Johnson
- Viatris Inc.
- GlaxoSmithKline
- Sandoz Inc. (Novartis)
- Lupin Pharmaceuticals, Inc.
- Sun Pharmaceutical Industries, Inc.
- Teva Pharmaceutical Industries Ltd.
Pulmonary Arterial Hypertension Market Segmentation
- By Drug Class
- Endothelin Receptor Antagonists (ERAs)
- PDE-5 Inhibitors
- Prostacyclin and Prostacyclin Analogs
- SGC Stimulators
- By Type
- Branded
- Generics
- By Route of Administration
- Oral
- Intravenous/ subcutaneous
- Inhalational
- Regional
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- France
- Italy
- Spain
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- MEA
- South Africa
- Saudi Arabia
- UAE
- North America
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Pulmonary Arterial Hypertension Market, By Drug Class
7.1. Pulmonary Arterial Hypertension Market, by Drug Class, 2021-2030
7.1.1. Endothelin Receptor Antagonists (ERAs)
7.1.1.1. Market Revenue and Forecast (2019-2030)
7.1.2. PDE-5 Inhibitors
7.1.2.1. Market Revenue and Forecast (2019-2030)
7.1.3. Prostacyclin and Prostacyclin Analogs
7.1.3.1. Market Revenue and Forecast (2019-2030)
7.1.4. SGC Stimulators
7.1.4.1. Market Revenue and Forecast (2019-2030)
Chapter 8. Global Pulmonary Arterial Hypertension Market, By Type
8.1. Pulmonary Arterial Hypertension Market, by Type, 2021-2030
8.1.1. Branded
8.1.1.1. Market Revenue and Forecast (2019-2030)
8.1.2. Generics
8.1.2.1. Market Revenue and Forecast (2019-2030)
Chapter 9. Global Pulmonary Arterial Hypertension Market, By Route of Administration
9.1. Pulmonary Arterial Hypertension Market, by Route of Administration, 2021-2030
9.1.1. Oral
9.1.1.1. Market Revenue and Forecast (2019-2030)
9.1.2. Intravenous/ subcutaneous
9.1.2.1. Market Revenue and Forecast (2019-2030)
9.1.3. Inhalational
9.1.3.1. Market Revenue and Forecast (2019-2030)
Chapter 10. Global Pulmonary Arterial Hypertension Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.1.2. Market Revenue and Forecast, by Type (2019-2030)
10.1.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.1.4. U.S.
10.1.4.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.1.4.2. Market Revenue and Forecast, by Type (2019-2030)
10.1.4.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.1.5. Rest of North America
10.1.5.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.1.5.2. Market Revenue and Forecast, by Type (2019-2030)
10.1.5.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.2.2. Market Revenue and Forecast, by Type (2019-2030)
10.2.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.2.4. UK
10.2.4.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.2.4.2. Market Revenue and Forecast, by Type (2019-2030)
10.2.4.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.2.5. Germany
10.2.5.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.2.5.2. Market Revenue and Forecast, by Type (2019-2030)
10.2.5.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.2.6. France
10.2.6.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.2.6.2. Market Revenue and Forecast, by Type (2019-2030)
10.2.6.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.2.7. Rest of Europe
10.2.7.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.2.7.2. Market Revenue and Forecast, by Type (2019-2030)
10.2.7.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.3.2. Market Revenue and Forecast, by Type (2019-2030)
10.3.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.3.4. India
10.3.4.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.3.4.2. Market Revenue and Forecast, by Type (2019-2030)
10.3.4.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.3.5. China
10.3.5.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.3.5.2. Market Revenue and Forecast, by Type (2019-2030)
10.3.5.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.3.6. Japan
10.3.6.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.3.6.2. Market Revenue and Forecast, by Type (2019-2030)
10.3.6.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.3.7. Rest of APAC
10.3.7.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.3.7.2. Market Revenue and Forecast, by Type (2019-2030)
10.3.7.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.4.2. Market Revenue and Forecast, by Type (2019-2030)
10.4.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.4.4. GCC
10.4.4.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.4.4.2. Market Revenue and Forecast, by Type (2019-2030)
10.4.4.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.4.5. North Africa
10.4.5.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.4.5.2. Market Revenue and Forecast, by Type (2019-2030)
10.4.5.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.4.6. South Africa
10.4.6.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.4.6.2. Market Revenue and Forecast, by Type (2019-2030)
10.4.6.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.4.7. Rest of MEA
10.4.7.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.4.7.2. Market Revenue and Forecast, by Type (2019-2030)
10.4.7.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.5.2. Market Revenue and Forecast, by Type (2019-2030)
10.5.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.5.4. Brazil
10.5.4.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.5.4.2. Market Revenue and Forecast, by Type (2019-2030)
10.5.4.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
10.5.5. Rest of LATAM
10.5.5.1. Market Revenue and Forecast, by Drug Class (2019-2030)
10.5.5.2. Market Revenue and Forecast, by Type (2019-2030)
10.5.5.3. Market Revenue and Forecast, by Route of Administration (2019-2030)
Chapter 11. Company Profiles
11.1. United Therapeutics Corporation
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Bayer
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Gilead Sciences, Inc.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Johnson & Johnson
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. Viatris Inc.
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. GlaxoSmithKline
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Sandoz Inc. (Novartis)
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Lupin Pharmaceuticals, Inc.
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Sun Pharmaceutical Industries, Inc.
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Teva Pharmaceutical Industries Ltd.
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
Glossary of Terms
Buy this Research Report study@ https://www.visionresearchreports.com/report/cart/39125
Contact Us:
Vision Research Reports
Call: +1 9197 992 333
