According to a research report “Regulatory Affairs Outsourcing Market (By Service: Legal Representation, Regulatory Consulting, Product Registration & Clinical Trial Application, Regulatory Writing & Publication, Others; By Category: Biologics, Drugs, Medical Devices; By End User: Medical Device Company, Biotechnology Company, and Pharmaceutical Company; By Indication: Neurology, Oncology, Immunology, Cardiology, Others) – Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2022 – 2030″ published by Precedence Research.
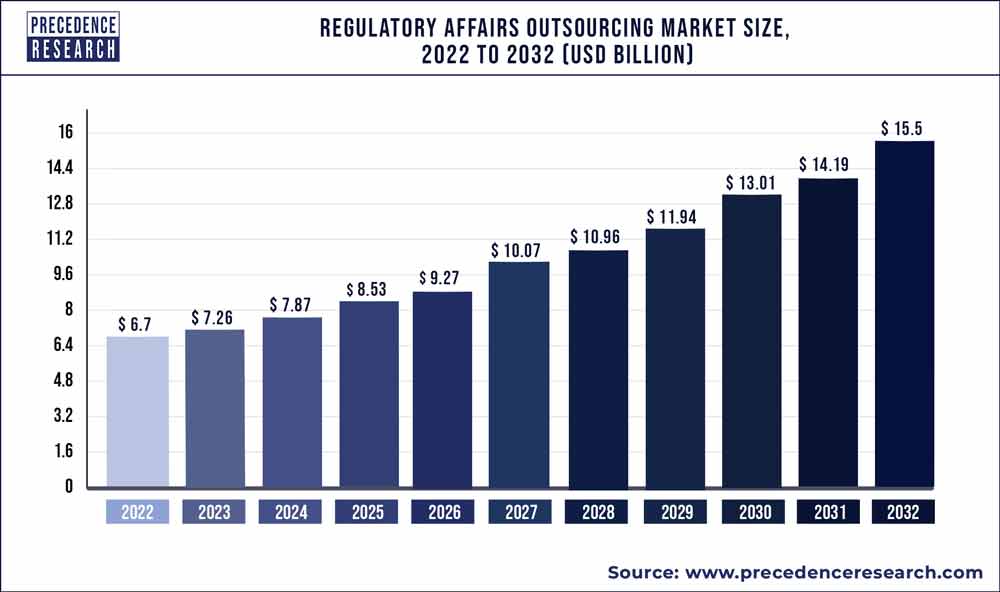
The regulatory affairs outsourcing market size is projected to touch USD 7.11 billion in 2022 and is forecasted to reach USD 16.6 billion by 2030; it is expected to grow at a CAGR of 10.2% from 2021 to 2030.
The study provides an analysis of the period 2017-2030, wherein 2021 to 2030 is the forecast period and 2021 is considered as the base year.
The global regulatory affairs outsourcing market is primarily driven by the globalization and the rising investments by the firms for the expansion. The increased research and development activities adopted by the players operating in the pharmaceutical and medical device industries augments the volume of clinical trials and product registration. This is a major driving force of the global regulatory affairs outsourcing market. The strict government regulations regarding the various medical products and complying to these stringent regulations in various countries is a major challenge to the drug and device manufacturers, which compels them to outsource the regulatory affairs to the third-party service providers.
Get a Free Sample Copy of this Report with Global Industry Analysis @ https://www.precedenceresearch.com/sample/1373
Asia Pacific was the dominating market in 2020. The increased presence of pharmaceutical and medical device manufacturers in the countries like China, India, South Korea, and Singapore is one of the primary and the most significant factors that propels the growth of the regulatory affairs outsourcing market. The easy and cheap availability of the factors of production in this region and the favorable government policies to attract FDIs are the major factors that attracts the global players to invest in this region. This rising number of production activities in this region requires a huge volume of regulatory affairs activities including product registration, regulatory writing, and clinical trials, which augments the market growth in this region.
The rising demand for the cost-reduction in the life sciences industry is augmenting the demand for the third party services for the regulatory affairs. Moreover, the introduction of various software that keeps the track of the various regulatory affairs is expected to provide lucrative growth opportunities to the market players in the foreseeable future.
The growing focus of the pharmaceutical companies and the medical devices manufacturing companies in the core activities coupled with the growing importance of complying to the regulatory standards is a major factors that is expected to drive the growth of the global regulatory affairs outsourcing market.
Report Scope of the Regulatory Affairs Outsourcing Market
| Report Highlights | Details |
| Market Size | USD 16.6 Billion by 2030 |
| Growth | CAGR of 10.2% From 2022 to 2030 |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Service, Category, End User, Indication, Stage |
| Regional Scope |
|
Based on service, the regulatory writing & publication segment dominated the market in 2020, garnering over 35% of the market share. The regulatory writing plays an essential role in communicating regarding the research and development works related to the new drugs and new medical devices developed by a company to the regulatory authorities like FDA. Regulatory writing is an integral part of the regulatory affairs activities, which is necessary for the acquisition of approvals in the concerned countries, and hence it captures the huge amount of the market share.
Based on the category, the biologics is estimated to be the most opportunistic segment during the forecast period. The rising investments in the development of various biologic medicines that provides an effective treatment for the chronic diseases like cancer, diabetes, and leukemia is a significant factor that propels the demand for the regulatory affairs outsourcing in order to get market authorization in various nations. Moreover, rising prevalence of chronic diseases among the population is expected to drive the growth of the biologics market, which in turn would propel the growth of the regulatory affairs outsourcing market in the foreseeable future.
Based on end user, the pharmaceutical company segment dominated the market, accounting for over 37% of the market share in 2020. The rapid growth of the biopharmaceuticals across the globe, and development of new and innovative medicines has fostered the growth in the volume of regulatory activities in the past few years. Moreover, the introduction of various blockbuster biopharmaceutical products and increased investments by the government and the private sector has played an influential role in the development of this segment.
Based on the indication, the neurology is estimated to be the most opportunistic segment during the forecast period. The rising prevalence of neurological disorders is boosting the need for the development of new drugs and medical devices. According to the Global Burden of Diseases report, around 95% people were suffering from the neurological disorders in the year 2016. Hence, rising investments in the new drug development for the neurological disorders is expected to foster the growth of this segment.
Based on the stage, the clinical segment dominated the market, accounting for more than 45% of the market share in 2020. The rising demand for the clinical trials among the drug developers and medical device manufacturers has augmented the market growth. According to the ClinicalTrials.gov, around 326,000 clinical trials were registered in 2019 in the US which increased to more than 347,000 clinical trials in 2020.
In 2019, Accell and Syntax collaborated to expand their client reach in the Europe.The various developmental strategies like collaborations, mergers, acquisitions, and partnerships fosters market growth and offers lucrative growth opportunities to the market players.
Read Also: Fuel Cell Market Size to Touch US$ 42.3 Bn by 2030
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Regulatory Affairs Outsourcing Market
5.1. COVID-19 Landscape: Regulatory Affairs Outsourcing Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Regulatory Affairs Outsourcing Market, By Service
8.1. Regulatory Affairs Outsourcing Market, by Service, 2021-2030
8.1.1. Legal Representation
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Regulatory Consulting
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Product Registration & Clinical Trial Application
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Regulatory Writing & Publication
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Others
8.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Regulatory Affairs Outsourcing Market, By Category
9.1. Regulatory Affairs Outsourcing Market, by Category, 2021-2030
9.1.1. Biologics
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Drugs
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Medical Devices
9.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Regulatory Affairs Outsourcing Market, By End User
10.1. Regulatory Affairs Outsourcing Market, by End User, 2021-2030
10.1.1. Medical Device Company
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Biotechnology Company
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Pharmaceutical Company
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Regulatory Affairs Outsourcing Market, By Indication
11.1. Regulatory Affairs Outsourcing Market, by Indication, 2021-2030
11.1.1. Neurology
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Oncology
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Immunology
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Cardiology
11.1.4.1. Market Revenue and Forecast (2017-2030)
11.1.5. Others
11.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Regulatory Affairs Outsourcing Market, By Stage
12.1. Regulatory Affairs Outsourcing Market, by Stage, 2021-2030
12.1.1. Clinical
12.1.1.1. Market Revenue and Forecast (2017-2030)
12.1.2. Preclinical
12.1.2.1. Market Revenue and Forecast (2017-2030)
12.1.3. Post Market Authorization
12.1.3.1.Market Revenue and Forecast (2017-2030)
Chapter 13. Global Regulatory Affairs Outsourcing Market, Regional Estimates and Trend Forecast
13.1. North America
13.1.1. Market Revenue and Forecast, by Service (2017-2030)
13.1.2. Market Revenue and Forecast, by Category (2017-2030)
13.1.3. Market Revenue and Forecast, by End User (2017-2030)
13.1.4. Market Revenue and Forecast, by Indication (2017-2030)
13.1.5. Market Revenue and Forecast, by Stage (2017-2030)
13.1.6. U.S.
13.1.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.1.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.1.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.1.6.4. Market Revenue and Forecast, by Indication (2017-2030)
13.1.7. Market Revenue and Forecast, by Stage (2017-2030)
13.1.8. Rest of North America
13.1.8.1. Market Revenue and Forecast, by Service (2017-2030)
13.1.8.2. Market Revenue and Forecast, by Category (2017-2030)
13.1.8.3. Market Revenue and Forecast, by End User (2017-2030)
13.1.8.4. Market Revenue and Forecast, by Indication (2017-2030)
13.1.8.5. Market Revenue and Forecast, by Stage (2017-2030)
13.2. Europe
13.2.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.4. Market Revenue and Forecast, by Indication (2017-2030)
13.2.5. Market Revenue and Forecast, by Stage (2017-2030)
13.2.6. UK
13.2.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.7. Market Revenue and Forecast, by Indication (2017-2030)
13.2.8. Market Revenue and Forecast, by Stage (2017-2030)
13.2.9. Germany
13.2.9.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.9.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.9.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.10. Market Revenue and Forecast, by Indication (2017-2030)
13.2.11. Market Revenue and Forecast, by Stage (2017-2030)
13.2.12. France
13.2.12.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.12.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.12.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.12.4. Market Revenue and Forecast, by Indication (2017-2030)
13.2.13. Market Revenue and Forecast, by Stage (2017-2030)
13.2.14. Rest of Europe
13.2.14.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.14.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.14.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.14.4. Market Revenue and Forecast, by Indication (2017-2030)
13.2.15. Market Revenue and Forecast, by Stage (2017-2030)
13.3. APAC
13.3.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.5. Market Revenue and Forecast, by Stage (2017-2030)
13.3.6. India
13.3.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.6.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.7. Market Revenue and Forecast, by Stage (2017-2030)
13.3.8. China
13.3.8.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.8.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.8.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.8.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.9. Market Revenue and Forecast, by Stage (2017-2030)
13.3.10. Japan
13.3.10.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.10.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.10.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.10.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.10.5. Market Revenue and Forecast, by Stage (2017-2030)
13.3.11. Rest of APAC
13.3.11.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.11.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.11.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.11.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.11.5. Market Revenue and Forecast, by Stage (2017-2030)
13.4. MEA
13.4.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.5. Market Revenue and Forecast, by Stage (2017-2030)
13.4.6. GCC
13.4.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.6.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.7. Market Revenue and Forecast, by Stage (2017-2030)
13.4.8. North Africa
13.4.8.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.8.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.8.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.8.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.9. Market Revenue and Forecast, by Stage (2017-2030)
13.4.10. South Africa
13.4.10.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.10.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.10.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.10.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.10.5. Market Revenue and Forecast, by Stage (2017-2030)
13.4.11. Rest of MEA
13.4.11.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.11.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.11.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.11.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.11.5. Market Revenue and Forecast, by Stage (2017-2030)
13.5. Latin America
13.5.1. Market Revenue and Forecast, by Service (2017-2030)
13.5.2. Market Revenue and Forecast, by Category (2017-2030)
13.5.3. Market Revenue and Forecast, by End User (2017-2030)
13.5.4. Market Revenue and Forecast, by Indication (2017-2030)
13.5.5. Market Revenue and Forecast, by Stage (2017-2030)
13.5.6. Brazil
13.5.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.5.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.5.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.5.6.4. Market Revenue and Forecast, by Indication (2017-2030)
13.5.7. Market Revenue and Forecast, by Stage (2017-2030)
13.5.8. Rest of LATAM
13.5.8.1. Market Revenue and Forecast, by Service (2017-2030)
13.5.8.2. Market Revenue and Forecast, by Category (2017-2030)
13.5.8.3. Market Revenue and Forecast, by End User (2017-2030)
13.5.8.4. Market Revenue and Forecast, by Indication (2017-2030)
13.5.8.5. Market Revenue and Forecast, by Stage (2017-2030)
Chapter 14. Company Profiles
14.1. Medpace
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. ICON Plc
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. WuXiAppTec, Inc.
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. Covance
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. Genpact Ltd.
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. Pharmaceutical Product Development LLC.
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. Freyr
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. PRA Health Sciences
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
14.9. Criterium, Inc.
14.9.1. Company Overview
14.9.2. Product Offerings
14.9.3. Financial Performance
14.9.4. Recent Initiatives
14.10. Accell Clinical Research, LLC.
14.10.1. Company Overview
14.10.2. Product Offerings
14.10.3. Financial Performance
14.10.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. Appendix
16.1. About Us
16.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Buy Full Research Report (Single User License US$ 4500) @ https://www.precedenceresearch.com/checkout/1373
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Website: https://www.precedenceresearch.com